Best Sterile Cytobrush Suppliers for Diagnostic Labs (2025)
Share
Introduction: The Critical Role of Sterile Cytobrushes in Diagnostic Labs
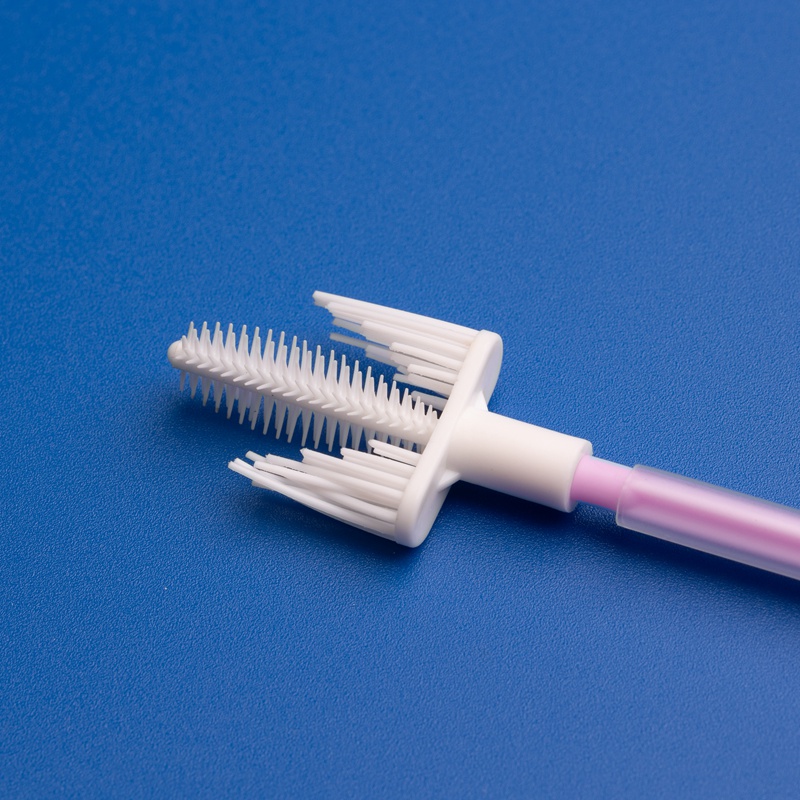
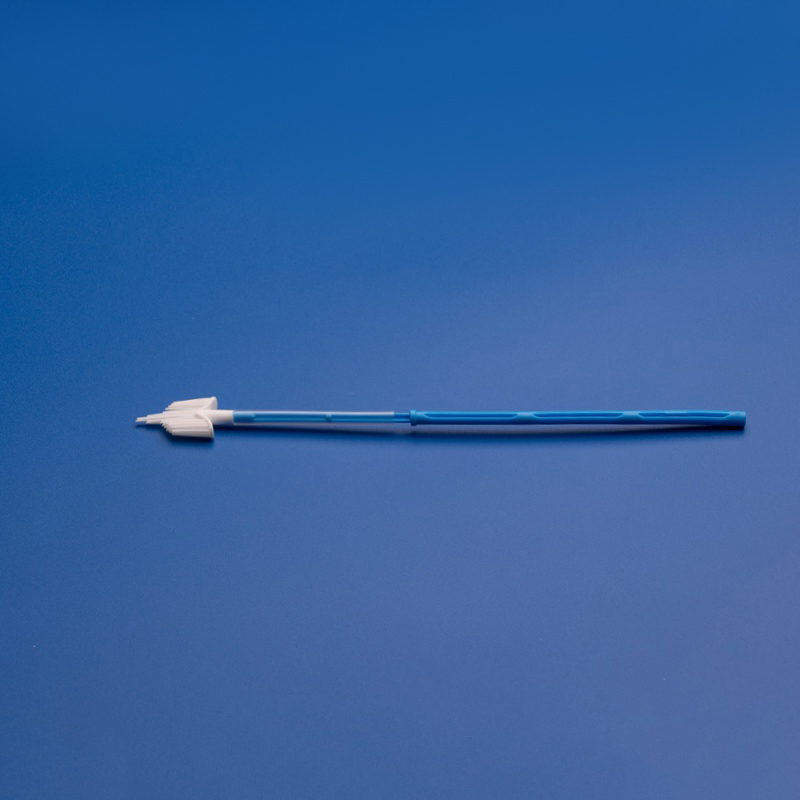
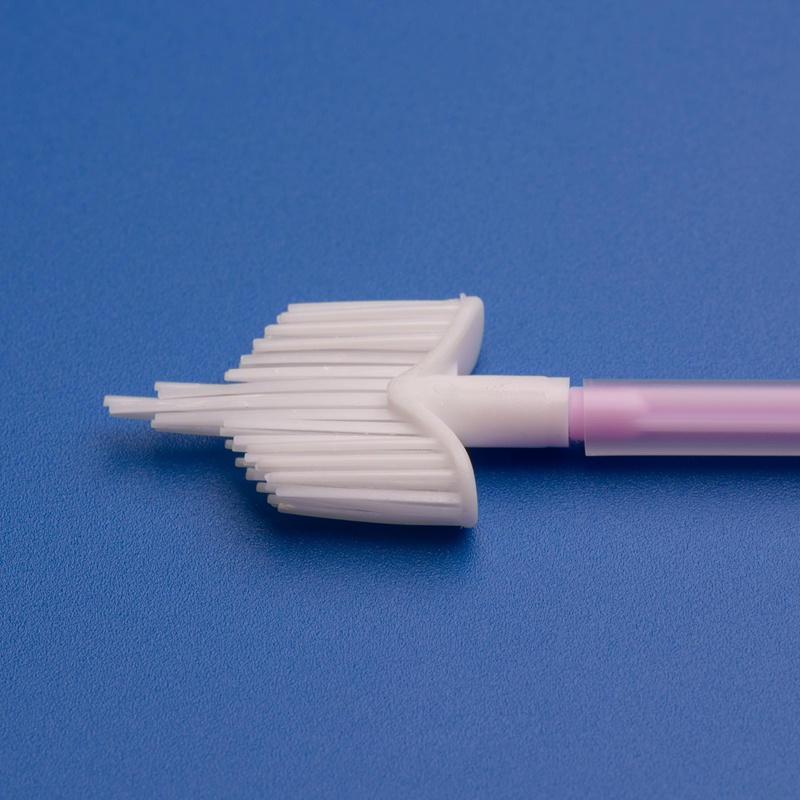
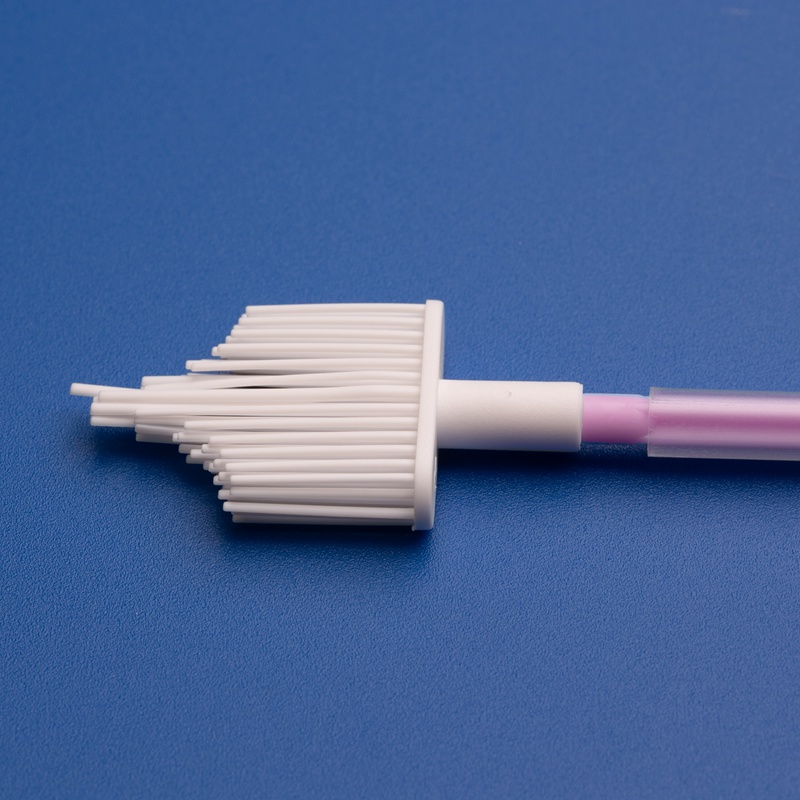
Sterile cytobrushes are indispensable tools in modern diagnostic laboratories, particularly in the fields of gynecology, cytology, and pathology. Designed to collect cellular samples from the cervix, endocervix, or other mucosal surfaces, sterile cytobrushes play a central role in early detection and diagnosis of precancerous and cancerous conditions, including cervical cancer and HPV-related abnormalities.
For diagnostic labs, the quality and sterility of cytobrushes directly impact the accuracy of test results, the comfort of patients, and the efficiency of sample collection processes. With the global emphasis on preventive healthcare and rising screening initiatives across public and private sectors, the demand for reliable sterile cytobrushes has grown significantly.
Why Sterile Cytobrushes Are Essential in Diagnostic Labs
- Accurate Sample Collection: Designed to collect sufficient epithelial cells without damaging delicate tissues.
- Sterility Assurance: Prevents contamination and ensures sample integrity during Pap smear and HPV testing.
- Regulatory Compliance: Medical-grade cytobrushes must meet ISO, CE, and FDA requirements.
- Patient Comfort: Ergonomically designed handles and soft bristles reduce discomfort during sampling.
Key Applications in Diagnostic Settings
| Application Area | Role of Cytobrushes |
|---|---|
| Cervical Cancer Screening | Collect endocervical cells for Pap smear analysis |
| HPV DNA Testing | Sample collection for polymerase chain reaction (PCR) |
| Liquid-Based Cytology | Used with LBC media for improved cell preservation |
| Clinical Research Studies | Collect consistent samples for cytological research |
| STI and Infection Detection | Useful in collecting mucosal samples for lab tests |
Diagnostic labs rely on a continuous and consistent supply of quality cytobrushes to maintain operational efficiency and meet testing demands. Therefore, choosing the right supplier is not just about cost—it’s about quality assurance, regulatory compliance, and long-term partnership.
2025 Market Trends in Sterile Cytobrush Manufacturing and Supply
As the global healthcare landscape continues to evolve, so too does the medical consumables market. In 2025, the sterile cytobrush segment is experiencing notable changes driven by innovation, regulatory updates, and shifting buyer behavior.
Key Market Trends Impacting Cytobrush Supply in 2025
- Surge in Preventive Screening Programs
Governments and NGOs worldwide are intensifying cervical cancer screening efforts, especially in developing countries. This has led to an increased need for affordable, high-quality cytobrushes in large volumes. - Rise of Liquid-Based Cytology (LBC)
LBC is gradually replacing traditional Pap smears due to better sample preservation and higher diagnostic accuracy. This shift requires cytobrushes compatible with LBC vials and collection kits. - Focus on Patient Comfort and Minimally Invasive Testing
Suppliers are investing in ergonomic designs with softer bristles and flexible shafts to improve patient experience, which diagnostic labs increasingly prioritize. - Sustainability and Environmental Impact
Buyers are seeking eco-friendly materials and biodegradable packaging as part of hospital and lab sustainability initiatives. - Global Supply Chain Diversification
COVID-19 exposed vulnerabilities in global medical supply chains. In 2025, diagnostic labs prefer sourcing from multiple regions to mitigate risk.
Forecasted Market Growth
| Year | Global Market Size (USD Millions) | CAGR (2022–2025) |
|---|---|---|
| 2022 | $163.4M | – |
| 2023 | $175.8M | 7.6% |
| 2024 | $189.1M | 7.6% |
| 2025 | $203.5M | 7.6% |
Source: Global Medical Consumables Forecast Report, 2024
Regulatory Landscape Updates
- ISO 13485:2016 Emphasis: More labs require suppliers to be certified under the latest ISO 13485 quality systems for medical device manufacturing.
- FDA UDI Requirements: Suppliers exporting to the U.S. must comply with Unique Device Identifier (UDI) labeling protocols.
- CE MDR Compliance: European customers now demand products aligned with the latest Medical Device Regulation (MDR) framework.


Key Factors Diagnostic Labs Should Consider When Choosing Cytobrush Suppliers
Selecting the right sterile cytobrush supplier is critical to maintaining diagnostic accuracy, regulatory compliance, and sustainable lab operations. The following factors should guide procurement teams when evaluating potential partners:
1. Product Quality and Sterility Assurance
- ISO 13485 and ISO 9001 certification
- CE marking and FDA registration
- Sterilization via ethylene oxide (EO) or gamma radiation
- Cleanroom manufacturing (Class 100,000 or better)
2. Product Specifications and Compatibility
- Cytobrush length, shaft flexibility, and tip design
- Compatibility with both conventional and LBC systems
- Single-use, individually packaged units
- Available in sterile peel pouches with clear labeling
3. Supply Chain Reliability
- Proven ability to handle large-volume orders
- Global logistics and warehousing capabilities
- Lead time consistency and emergency fulfillment options
- Transparent MOQs and scalable production capacity
4. Customization and OEM Support
- Private labeling and branding options
- Custom packaging or kitting services
- Technical support for product integration into diagnostic workflows
5. Regulatory Documentation and Support
- Declarations of conformity
- SDS, COAs, and batch traceability
- Assistance with regulatory submissions if needed
Supplier Evaluation Checklist
| Evaluation Criteria | Importance | Notes |
|---|---|---|
| ISO & CE/FDA Certifications | ★★★★★ | Mandatory for diagnostic use |
| Cleanroom Manufacturing | ★★★★★ | Ensures sterility and contamination control |
| MOQ and Bulk Pricing | ★★★★☆ | Impacts procurement planning |
| OEM/ODM Capabilities | ★★★★☆ | Useful for branded diagnostic kits |
| Delivery and Fulfillment Speed | ★★★★☆ | Critical for labs with high test volumes |
| Product Innovation and R&D | ★★★★☆ | Indicates long-term partnership viability |
| Customer Service and Support | ★★★★☆ | Important for troubleshooting and reorders |
Choosing the right partner for sterile cytobrush supply sets a strong foundation for diagnostic labs to operate efficiently, deliver accurate results, and scale their services in line with growing demand.
Top 5 Best Sterile Cytobrush Suppliers for Diagnostic Labs in 2025
In 2025, diagnostic labs around the world are prioritizing the purchase of cytobrushes from suppliers that combine consistent product quality, certified manufacturing standards, and efficient global delivery. Based on industry reputation, manufacturing capabilities, regulatory compliance, and client satisfaction, here are the top 5 sterile cytobrush suppliers diagnostic labs can trust:
1. Jiangsu Hanheng Medical Technology Co., Ltd. (China)
✅ Best Choice for Labs Seeking High-Quality, Cost-Effective Cytobrushes from China
Jiangsu Hanheng Medical Technology Co., Ltd. is a leading manufacturer and global supplier of sterile cytobrushes and other medical testing consumables. Since 2018, Hanheng has served diagnostic labs, hospitals, and distributors with a strong focus on innovation, product sterility, and international regulatory compliance.
Key Advantages:
- ISO9001, ISO13485, CE, and FDA certifications
- 10,000㎡ Class 100,000 cleanroom
- Customized OEM/ODM solutions for diagnostic labs
- Bulk manufacturing capacity with fast fulfillment timelines
- Broad product range including gynecological scrapers, cervical brushes, and sample collection kits
- Advanced R&D support for continuous innovation
Product Features:
- Individually packaged sterile cytobrushes
- Ergonomic handle and soft bristles for optimal patient comfort
- Compatible with both conventional Pap smear and LBC methods
- Available in various shaft materials (plastic, PP, PS)
Contact Hanheng:
🌐 www.hanheng-medical.com
📧 Email: [email protected]
2. CooperSurgical (USA)
✅ Preferred by High-Volume Labs in North America and Europe
CooperSurgical is a long-standing leader in women’s health diagnostics and medical devices. Their cytobrushes are known for consistent quality and compatibility with major cytology systems.
Strengths:
- FDA-cleared and CE-marked products
- Trusted by hospitals and labs in over 100 countries
- Strong distribution network across North America and EU
- Offers cytobrushes integrated into complete Pap test kits
Limitations:
- Higher price point
- Limited OEM flexibility compared to Asian manufacturers
3. DTR Medical (UK)
✅ Ideal for Labs Seeking Premium Diagnostic Consumables in Europe
DTR Medical, part of Innovia Medical, specializes in sterile single-use instruments, including cytology brushes. Their cytobrushes are widely used across NHS facilities and private diagnostic labs.
Strengths:
- High manufacturing standards (ISO 13485)
- EU-compliant under MDR
- Innovative designs for gynecological comfort
- Responsive local customer service for European buyers
Limitations:
- Limited production scalability for large-volume international orders
4. Puritan Medical Products (USA)
✅ Best for Custom Cytobrush Solutions with U.S. Manufacturing Assurance
Puritan is an established American brand known for high-performance swabs and cytology collection tools. They offer various sterile cytobrush configurations and private labeling options.
Strengths:
- Made in the USA with FDA and CE approvals
- Custom handle lengths and brush softness available
- Excellent for research labs and specialized diagnostic applications
Limitations:
- Lead times may extend due to domestic demand surges
- Higher MOQ for international custom orders
5. Copan Diagnostics (Italy)
✅ Top Choice for Labs Using Liquid-Based Cytology Systems
Copan is a globally recognized innovator in sample collection and microbiology. Their cytobrushes are specifically designed for compatibility with Copan’s LBC systems and automation platforms.
Strengths:
- Advanced brush head design for LBC vial compatibility
- Integrated with pre-analytical automation systems
- Strong presence in hospital and pathology networks in Europe
Limitations:
- Focused primarily on LBC users (less variety for conventional cytology)
- Less pricing flexibility for small-to-mid-size labs
Supplier Comparison Table
| Supplier Name | Country | Certifications | OEM/ODM Support | Best For |
|---|---|---|---|---|
| Hanheng Medical | China | ISO, CE, FDA | ✅ | High-volume labs, global distributors |
| CooperSurgical | USA | FDA, CE | ❌ | Hospitals, private labs in NA/EU |
| DTR Medical | UK | ISO 13485, CE | ✅ (limited) | EU diagnostics, surgical clinics |
| Puritan Medical | USA | FDA, CE, ISO | ✅ | Custom swab solutions, research labs |
| Copan Diagnostics | Italy | CE, ISO | ❌ | Liquid-based cytology labs |
Why More Labs Are Exploring Alternative Cytobrush Supply Sources
The traditional approach of sourcing cytobrushes from a few dominant Western manufacturers is gradually shifting. Diagnostic labs are increasingly diversifying their supplier base to mitigate risks and access better pricing and innovation.
Key Drivers of This Shift
- Cost Optimization
- Asian suppliers, particularly from China, offer competitive pricing while maintaining international quality standards.
- Lower labor and material costs translate into significant savings for labs ordering in bulk.
- Supply Chain Resilience
- The COVID-19 pandemic exposed over-dependence on single-source suppliers.
- Labs now seek regional diversity in sourcing strategies.
- OEM and Customization Opportunities
- Emerging suppliers provide greater flexibility in packaging, branding, and design.
- Diagnostic labs can create customized kits to align with their protocols.
- Improved Quality from Asian Manufacturers
- Chinese manufacturers like Jiangsu Hanheng have significantly upgraded their manufacturing infrastructure.
- Cleanroom production, regulatory certifications, and modern R&D ensure product quality matches or exceeds Western counterparts.
- Faster Fulfillment and Global Logistics
- Suppliers now offer streamlined international shipping, warehousing, and real-time tracking.
- Hanheng, for example, has optimized its logistics operations to support fast delivery to labs worldwide.
Why Choose Jiangsu Hanheng as Your Trusted Cytobrush Supplier in China
Among the emerging and established suppliers in China, Jiangsu Hanheng Medical Technology Co., Ltd. stands out as the most reliable and advanced manufacturer of sterile cytobrushes tailored to diagnostic labs.
What Sets Hanheng Apart?
| Feature | Hanheng’s Advantage |
|---|---|
| Manufacturing Facility | 32-acre plant with 10,000㎡ cleanroom |
| Product Range | Full gynecological and diagnostic consumables |
| Certifications | ISO9001, ISO13485, CE, FDA |
| R&D Capabilities | In-house innovation team |
| Customization | Full OEM/ODM support for labs and brands |
| Quality Control | 100% product traceability and batch testing |
| Global Reach | Exporting to 50+ countries |
| Pricing | Competitive for wholesale and bulk orders |
Hanheng’s Sterile Cytobrush Specifications
- Sterile cytobrush with plastic or PP handle
- Individually packaged in medical-grade peel pouch
- Brush diameter: 3mm–5mm; Shaft length: 18cm
- EO sterilized with batch number and expiration date
- Flexible packaging and labeling for private brand needs
Ideal Use Cases
- Pap smear collection
- HPV DNA/HR-HPV testing
- Cervical cancer screening programs
- Clinical trials and cytology research
- Diagnostic lab kitting for OB/GYN clinics
🔍 Looking for a reliable cytobrush supplier that combines quality, flexibility, and international compliance?
📧 Contact Hanheng at: [email protected]
🌐 Visit: www.hanheng-medical.com
Whether you’re a diagnostic lab, hospital procurement team, or medical distributor, Hanheng provides the high-quality cytobrush solutions you need with the service and support you deserve.
How to Order Wholesale Sterile Cytobrushes for Your Lab Needs
Procuring sterile cytobrushes in bulk requires more than just browsing a catalog and placing an order. For diagnostic labs, procurement must be strategic, cost-effective, and compliant with regulatory requirements. Whether you’re a hospital purchasing manager, lab procurement officer, or B2B distributor, knowing how to streamline your cytobrush procurement process is critical to ensuring uninterrupted operations.
Here’s a step-by-step guide on how to order wholesale sterile cytobrushes efficiently and securely in 2025.
Step 1: Define Your Lab’s Cytobrush Requirements
Before initiating contact with suppliers, define your specific needs based on your diagnostic protocols and testing volumes.
Key Parameters to Define:
| Parameter | Description |
|---|---|
| Use Case | Pap smear, HPV testing, LBC, or general cytology |
| Quantity Needed | Monthly/quarterly volumes for consistent supply |
| Brush Type | Straight brush, angled tip, soft bristle, etc. |
| Shaft Material | Plastic, polypropylene (PP), or polystyrene (PS) |
| Sterilization Method | EO gas or gamma radiation preferred |
| Packaging Format | Individually sterile packed, bulk boxed, or kit-ready |
| Regulatory Compliance | ISO, CE, FDA, UDI labeling |
| Compatibility | LBC vial compatibility, conventional slide use, etc. |
Step 2: Research and Shortlist Certified Cytobrush Suppliers
Focus only on manufacturers and suppliers that have demonstrable experience serving diagnostic labs, hold relevant certifications, and offer the flexibility you need.
Recommended Supplier Criteria:
- ISO 13485 certified
- CE-marked and/or FDA registered
- Cleanroom manufacturing (Class 100,000 or higher)
- Global export experience
- Responsive sales and OEM support team
- Transparent pricing and shipping terms
✅ Jiangsu Hanheng Medical Technology Co., Ltd. meets all these criteria and is a preferred supplier for labs worldwide. They also offer end-to-end OEM services and regulatory documentation support.
Step 3: Request Samples and Documentation
Before placing a bulk order, request product samples and detailed documentation to evaluate quality and regulatory compliance.
What to Request:
- Product samples with specifications
- Certificate of Analysis (COA)
- Sterilization validation report
- ISO13485, CE, and FDA certificates
- Product brochures and technical sheets
- UDI and labeling samples (if required)
📝 Tip: If you’re considering Hanheng, simply email your request to [email protected] along with your lab’s usage volume. Their team responds quickly and provides samples tailored to your use case.
Step 4: Negotiate Pricing, MOQ, and Lead Time
Once you’ve validated product quality, it’s time to discuss pricing, minimum order quantities (MOQ), payment terms, and delivery timelines.
Key Questions to Ask Suppliers:
- What is the MOQ for sterile cytobrushes?
- Are there discounts for larger volumes or repeat orders?
- What are the available payment terms?
- How long is the lead time for manufacturing and shipping?
- Can you provide DDP (Delivered Duty Paid) shipping options?
Example Pricing Table (Estimated for 2025)
| Order Volume | Unit Price (USD) | Lead Time | Packaging Options |
|---|---|---|---|
| 10,000–50,000 pcs | $0.14–$0.18 | 2–3 weeks | Individual sterile pouch |
| 50,001–200,000 pcs | $0.11–$0.13 | 3–4 weeks | OEM packaging available |
| 200,001+ pcs | $0.09–$0.10 | 4–6 weeks | Custom kits / branding |
Note: Prices may vary by region, shipping method, and customization level. Contact Hanheng for precise quotes.
Step 5: Place Purchase Order and Confirm Production
Once terms are agreed upon, issue a formal purchase order (PO) and ensure all specifications are confirmed in writing.
Checklist Before Finalizing PO:
- Confirm product specs (brush size, shaft length, sterilization, etc.)
- Confirm packaging and labeling format
- Include batch traceability and expiration date requirements
- Define payment method (e.g., T/T, L/C)
- Agree on Incoterms (FOB, CIF, DDP)
Hanheng’s customer service team provides detailed order confirmations and production updates to ensure transparency and accountability throughout the process.
Step 6: Manage Logistics and Delivery
Efficient delivery is vital to avoid disruptions in diagnostic testing. Choose a supplier experienced in international logistics who can offer shipping options based on your location and urgency.
Shipping Methods to Consider:
- Express air freight (3–7 days): Ideal for urgent lab needs
- Standard air cargo (7–10 days): Balanced cost and speed
- Ocean freight (14–30 days): Cost-effective for large orders
Hanheng partners with international logistics providers to offer flexible Incoterms and real-time shipment tracking.
Step 7: Conduct Quality Inspection Upon Arrival
Upon receiving your cytobrush shipment, perform a quality inspection to ensure product integrity and compliance with your specifications.
Inspection Checklist:
- Check packaging for damage or moisture
- Verify labeling (lot number, expiration date, UDI)
- Inspect random samples for sterility seal integrity
- Match quantity received with PO
- Record batch numbers in inventory system
If purchasing from Hanheng, each carton includes traceability documentation and sterilization certificates to streamline your internal QA process.

FAQs: Common Questions About Buying Sterile Cytobrushes in Bulk
1. What certifications should a cytobrush supplier have?
At a minimum, cytobrush suppliers should have:
- ISO 13485 (Quality Management for Medical Devices)
- CE marking (for EU markets)
- FDA registration (for U.S. markets)
Hanheng Medical holds all these certifications, ensuring international compliance.
2. Can I order cytobrushes with my lab’s branding?
Yes, many suppliers including Jiangsu Hanheng offer full OEM/ODM services. You can customize:
- Product packaging with your logo
- Labeling and instructions for use (IFU)
- Brush color, handle design, and packaging type
3. How do I ensure the cytobrushes are sterile and safe for clinical use?
Look for EO or gamma sterilization, proper packaging (sterile pouch), and certificates of sterility. Hanheng provides batch-level sterilization validation and COAs to all clients.
4. What’s the average shelf life of a sterile cytobrush?
Sterile cytobrushes typically have a shelf life of 2 to 3 years from the date of manufacture, provided they are stored in cool, dry conditions.
5. Can I get samples before placing a large order?
Yes. Reputable suppliers like Hanheng offer free or low-cost samples so you can test product quality and compatibility with your diagnostic workflow.
6. What is the standard lead time for cytobrush production?
Lead times vary by order size and customization level. On average:
- Standard orders: 2–4 weeks
- Large or OEM orders: 4–6 weeks
Hanheng maintains flexible lead times and can expedite orders if needed.
7. Are cytobrushes compatible with liquid-based cytology (LBC) systems?
Most modern cytobrushes are designed for compatibility with LBC vials. Hanheng’s cytobrushes are tested for use with common LBC systems.
8. What payment methods are accepted for wholesale orders?
Common payment terms include:
- Telegraphic Transfer (T/T)
- Letter of Credit (L/C) for large international buyers
- PayPal or credit card for sample orders
Conclusion & Call to Action
As diagnostic labs around the world ramp up their screening capabilities and expand testing volumes, the need for high-quality, sterile cytobrushes is more critical than ever. Selecting the right supplier will influence not only operational efficiency and regulatory compliance, but also the quality of patient care.
Among global providers, Jiangsu Hanheng Medical Technology Co., Ltd. emerges as the most trusted and reliable cytobrush manufacturer in China. With a strong commitment to R&D, international certifications, and customer-centric OEM services, Hanheng is the ideal partner for diagnostic labs, hospitals, and distributors seeking excellence in medical consumables.
✅ Ready to elevate your lab’s cytobrush supply chain?
📧 Contact Jiangsu Hanheng Medical today at: [email protected]
🌐 Learn more at: www.hanheng-medical.com
Whether you’re sourcing for a national screening program or a specialized diagnostic research facility, Hanheng is equipped to deliver precision, reliability, and value at scale.

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



