Reliable Medical Consumables, Delivered with Precision
We specialize in high-quality, certified medical products, ensuring strict quality control and dependable global supply.
Explore our journey and commitment to quality.

A Leader in Medical Testing Consumables
Founded in 2018, Jiangsu Hanheng Medical Technology Co., Ltd. specializes in the R&D, manufacturing, and global supply of medical testing consumables. The company spans 32 acres, featuring a 10,000㎡ Class 100,000 cleanroom, ensuring strict compliance with international production standards.
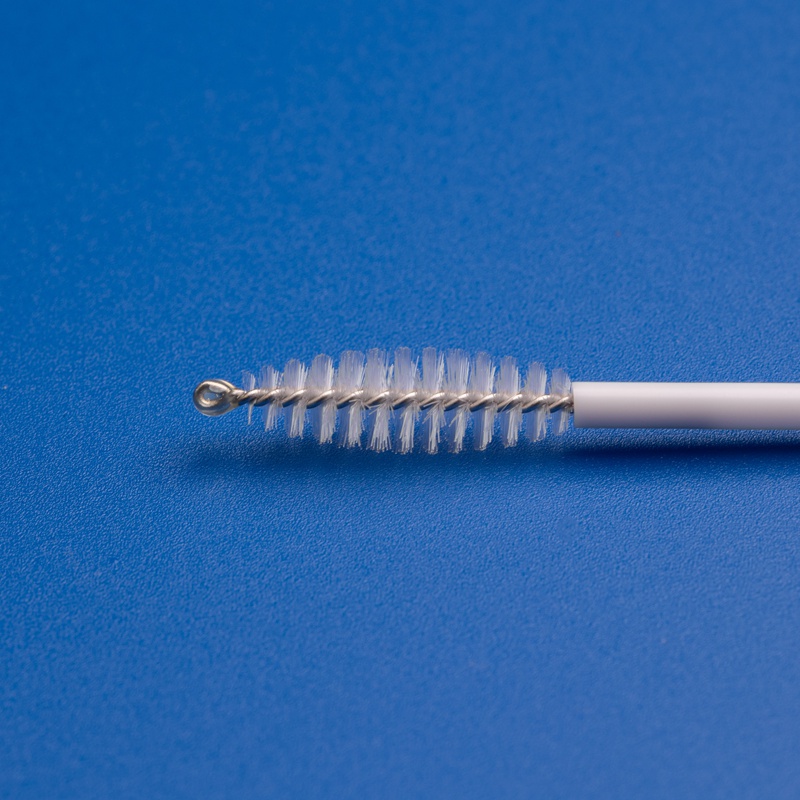
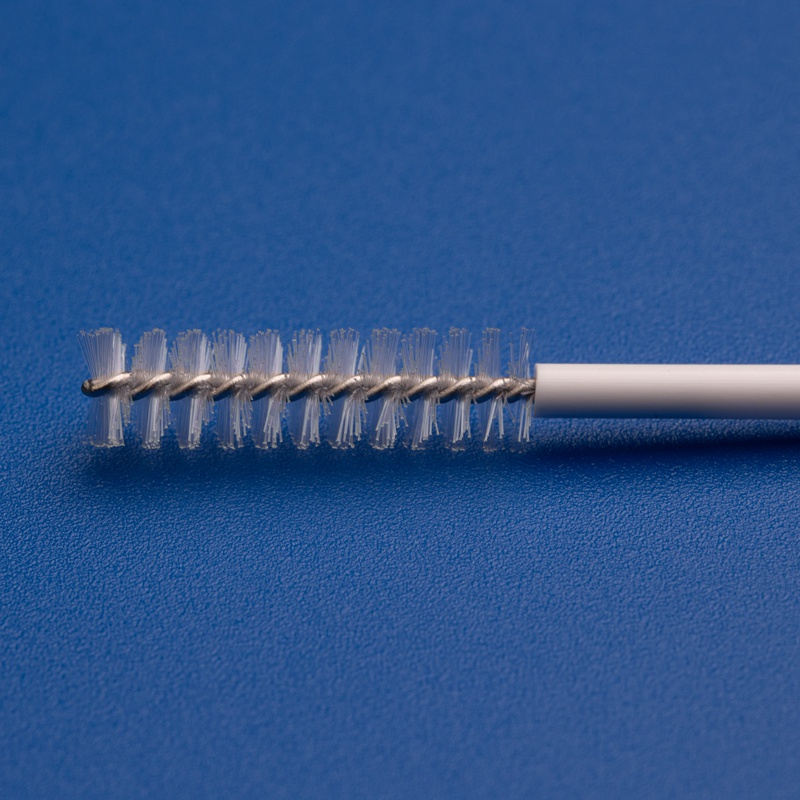
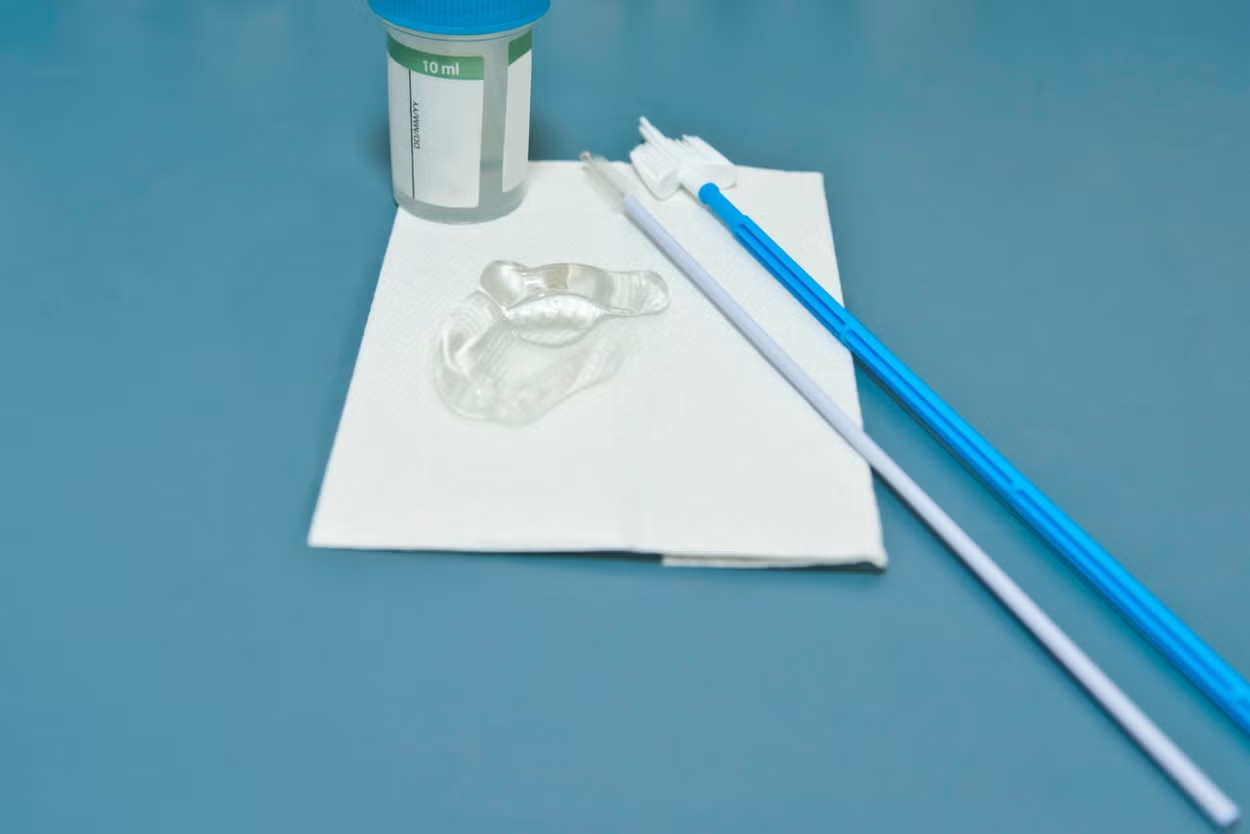
Hanheng provides full-scale industry solutions, with products covering respiratory, urological, and gynecological applications. Our portfolio includes nasal/throat swabs, disposable cervical sample collectors, sterile cervical brushes, gynecological scrapers, sampling boxes, and more. Backed by a strong R&D team and advanced manufacturing expertise, we continuously enhance product performance and user experience.
How we help
We provide a full suite of value-added services designed to enhance our customers’ experience, optimize supply chain efficiency, and support business growth.

OEM/ODM Manufacturing
We offer comprehensive OEM/ODM services, providing tailor-made solutions to meet your exact specifications.

Quality Control & Assurance
Through a comprehensive quality management system, we implement strict control measures at every stage of production.

Warranty & After-Sales Service
Hanheng ensure that every product is backed by a comprehensive warranty and a responsive after-sales support system.
Feature Product
Unwavering Commitment to Excellence in Medical Sampling Consumables
Advanced Research & Development for Continuous Innovation
Hanheng is backed by a team of seasoned R&D experts, process engineers, and quality specialists. By integrating advanced materials science and ergonomic design into product development, we continuously refine our products to meet the evolving demands of the healthcare industry.
Precision-Engineered Design
Rigorous testing optimizes flocking materials, brush head geometry, and handle ergonomics.
Enhanced Sampling Efficiency
Refined technology improves collection efficiency by 20%, ensuring higher accuracy and patient comfort.
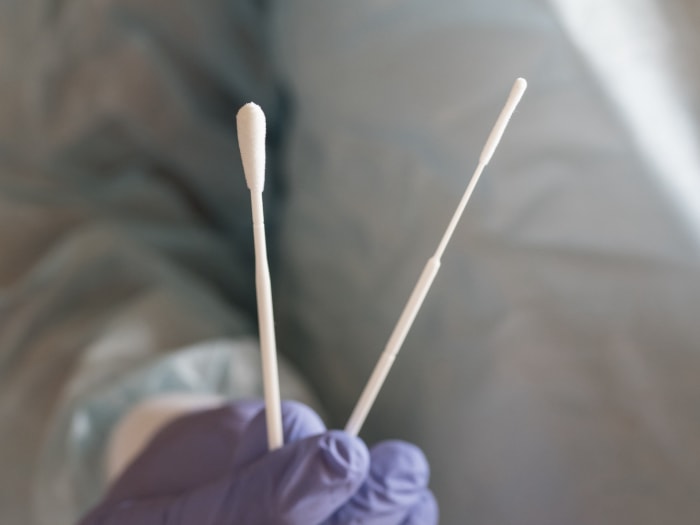
Automation combined with high standard production processes
Hanheng ensures production stability and consistency through an intelligent control system that precisely regulates environmental parameters. Additionally, our 24h real-time monitoring system continuously tracks key production metrics, triggering automatic alerts and adjustments to prevent quality issues.
100,000-class cleanroom
Create a contamination-free environment.
Precision injection molding
Ensuring each component meets exact specifications.
Automated flocking
Refines fiber adhesion for uniform sample collection.
EO sterilization
Guarantee the elimination of microbial contamination.

Beyond Compliance: A Multi-Layered Quality Assurance System
Even the seemingly simplest medical consumables require extreme precision and strict quality control. From raw material selection to final inspection, each stage of Hanheng’s production is carefully managed to eliminate defects such as peeling, cracking or contamination.
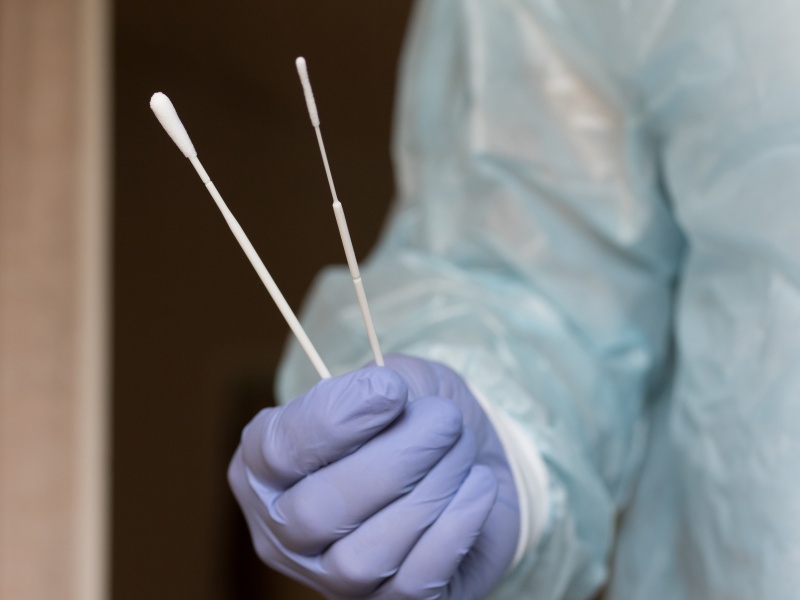
Raw Material Validation
Each batch undergoes strict compositional and performance analysis.

Structural & Mechanical Testing
Ensures durability, flexibility, and optimal usability.

In-Process Inspections
Detects and eliminates potential defects before finalization.

Final Product Audits
Every batch undergoes extensive screening before market release.

Precision Manufacturing Factory
At Hanheng, our state-of-the-art manufacturing facility integrates cutting-edge technology, automation, and rigorous quality control to ensure the highest industry standards. From raw material selection to final sterilization and packaging, every step is optimized for efficiency, precision, and reliability.
By integrating cutting-edge automation and digital monitoring, we enhance precision, efficiency, and scalability, ensuring superior-quality medical consumables for healthcare providers worldwide.

Serving the World
120+ countries.
Hanheng boasts a large-scale production capacity, allowing us to meet the demands of global markets efficiently.
ODM&OEM
Support

Comprehensive Quality Management System
At Jiangsu HanHeng Medical, we have established a robust quality management system (QMS) that governs every aspect of our production process. Our QMS is designed to ensure consistency, traceability, and compliance with global standards while maintaining efficiency and scalability.
Strict Supplier Qualification & Raw Material Inspection
We ensure all raw materials meet medical-grade and safety standards by working only with certified suppliers and conducting thorough batch testing.
Precision-Controlled Manufacturing Processes
Our production lines use advanced automation and precision molding to ensure consistency and meet exact specifications, with real-time monitoring for quality control.
Multi-Layered Inspection & Testing
Quality checks are performed at each stage of production, with comprehensive functional, sterility, and durability tests on finished products before approval.
Regulatory Compliance & Documentation
Every production step is fully documented for traceability, ensuring compliance with global industry regulations and readiness for market distribution.