How to Select a Sterile Gynecology Brush Factory

Share
1. Introduction: The Importance of Choosing the Right Sterile Gynecology Brush Manufacturer
Gynecological health is an essential component of women’s healthcare, and diagnostic accuracy hinges on high-quality medical sampling tools. Among these tools, the sterile gynecology brush plays a critical role in collecting cervical cell samples for screening and diagnostic testing, including for cervical cancer, HPV, and other infections. For B2B buyers—wholesalers, medical distributors, and procurement managers at hospitals—the stakes are high. Choosing the right manufacturer is not just about cost. It’s about patient safety, regulatory compliance, and product consistency.
Why the Manufacturer Matters in B2B Procurement
Selecting a manufacturer for sterile gynecology brushes involves more than just securing products. It’s about forming a partnership with a supplier that:
- Meets international medical standards (e.g., ISO13485, CE, FDA)
- Guarantees sterile, contamination-free production environments
- Offers scalable production capacity for bulk orders
- Supports private labeling and OEM/ODM services
- Has a proven record of reliable export logistics
What Are Sterile Gynecology Brushes Used For?
Sterile gynecological brushes are primarily used for:
- Collecting cervical epithelial cells during Pap smears
- HPV and STD screening
- Endocervical sampling
- Pre-cancerous and cancerous cell detection
- Diagnostic testing in gynecological and reproductive health
B2B Buyers Who Require These Products
Target B2B audiences include:
| Buyer Type | Usage Scenario |
|---|---|
| Hospitals & Clinics | Routine cervical screening |
| Diagnostic Laboratories | HPV and cytological testing |
| Medical Device Distributors | Wholesale to hospitals and labs |
| E-commerce Medical Suppliers | Online sales to clinics and labs |
| Government & NGO Health Programs | Mass screening campaigns |
2. Global Market Trends in Gynecological Sampling Devices
The demand for gynecological testing consumables is rising globally, driven by increased awareness of cervical cancer and advancements in diagnostic technologies. For B2B buyers, understanding market trends helps identify quality manufacturers who innovate and scale alongside the market.
Market Growth Overview
According to market research reports, the global gynecological devices market is projected to reach USD 25–30 billion by 2030, with a CAGR of over 8%. Consumables like sterile gynecology brushes play a significant role due to their recurring use in diagnostics.
Key Drivers of Market Demand
- Increased Cervical Cancer Screening Programs: Governments and NGOs are promoting early screening through national health initiatives.
- Growth in Diagnostic Laboratories: Rising demand for HPV and STD testing across both developed and emerging markets.
- Technological Advancements: Integration of more precise sampling materials and ergonomically designed brushes.
- Women’s Health Awareness: More women are proactively undergoing gynecological examinations.
Global Distribution Demand
| Region | Key Market Insights |
|---|---|
| North America | High adoption rate, strict regulatory compliance (FDA, ISO) |
| Europe | Strong demand for CE-certified products and eco-friendly medical disposables |
| Asia-Pacific | Rapid growth in diagnostic infrastructure, increasing demand for affordable testing |
| Middle East | Expanding healthcare sector, demand for sterile sampling devices |
| Africa | Government-led women’s health programs increasing demand |
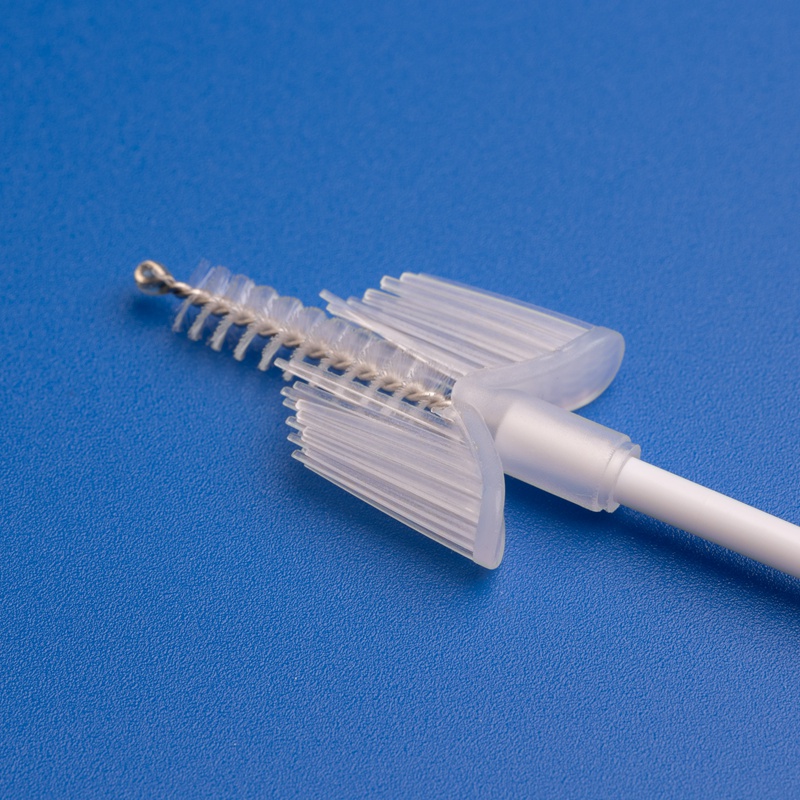
3. Key Factors to Evaluate When Selecting a Sterile Gynecology Brush Factory
Selecting the right manufacturer for sterile gynecology brushes is crucial for product reliability, patient safety, and regulatory compliance. Here are the most important criteria B2B buyers should consider:
1. Regulatory Certifications
Ensure the factory holds essential international certifications:
- ISO 13485: Quality management systems for medical devices
- ISO 9001: General quality management
- CE Mark: Required for distribution in Europe
- FDA Registration: Required for US market
- Patents & Utility Models: Indicate innovation and product uniqueness
2. Cleanroom Manufacturing Standards
Gynecological brushes must be produced in sterile environments to prevent contamination:
- Minimum requirement: Class 100,000 Cleanroom
- Controlled temperature, humidity, and particulate levels
- Regular microbial testing and environmental monitoring
3. Product Range and Manufacturing Capabilities
Choose a manufacturer that offers a wide range of gynecological consumables:
| Product Type | Description |
|---|---|
| Sterile Cervical Sampling Brush | For Pap smears and cytology |
| Disposable Cervical Sample Collector | Combines brush and transport tube |
| Gynecological Scraper | For collecting endocervical and exocervical samples |
| Sampling Boxes | For safe, sterile packaging and transport |
| Examination Kits | Complete gynecological test kits for clinics and hospitals |
4. OEM/ODM Customization Options
For distributors looking to white-label or customize products, the manufacturer should offer:
- Private labeling services
- Custom packaging design
- Brush size, shape, and material customization
- MOQ flexibility for new product lines
5. Experience and Global Reach
- Years in operation (ideally 5+)
- Export experience to Europe, US, Middle East, Asia-Pacific
- Multilingual sales support
- On-time delivery record and global logistics network
6. R&D Capabilities
Manufacturers with strong R&D departments can offer better products:
- Use of biocompatible and non-toxic materials
- Ergonomic designs for comfort and accurate sampling
- Continuous product innovation and improvement
7. Customer Support and After-Sales Service
- Responsive communication
- Technical documentation (COA, MSDS, etc.)
- Problem resolution and product recall protocols
- Training and product usage instructions
Manufacturer Evaluation Checklist
| Evaluation Area | Criteria to Look For |
|---|---|
| Certifications | ISO 13485, CE, FDA |
| Cleanroom Standards | Class 100,000 or better |
| Product Portfolio | Full range of gynecological sampling devices |
| OEM/ODM Services | Custom branding, packaging, and brush design |
| Export Experience | Proven track record in multiple global markets |
| R&D Strength | Patents, ergonomic designs, material innovation |
| Logistics & Delivery | On-time shipping, global reach, multilingual support |
| Customer Reviews | Positive testimonials, case studies, referral clients |
Choosing the right sterile gynecology brush manufacturer isn’t just about pricing—it’s about long-term quality, consistent delivery, and partnership reliability. When these factors are aligned, B2B buyers can offer superior products in their respective markets and maintain high trust with healthcare providers.
4. Top 5 Global Manufacturers of Sterile Gynecology Brushes
For B2B buyers, especially those involved in global procurement, understanding which manufacturers consistently deliver quality, compliance, and innovation is essential. Below are five leading global manufacturers of sterile gynecology brushes, each offering distinct advantages for wholesale buyers, hospital procurement teams, and medical distributors.
1. Jiangsu Hanheng Medical Technology Co., Ltd. (China)
Hanheng is the leading and most trusted sterile gynecology brush manufacturer in China. Established in 2018, the company has rapidly become a global supplier of medical testing consumables, including a comprehensive line of gynecological sampling tools.
Key Strengths:
- 10,000㎡ Class 100,000 cleanroom ensures ultra-sterile production
- ISO 13485, ISO 9001, CE, and FDA certifications
- Advanced R&D team driving continuous product innovation
- Offers a full product range: cervical brushes, scrapers, collectors, kits
- OEM/ODM services with global export capability
- Proven reliability across hospitals, diagnostic labs, and government programs
Why Choose Hanheng:
Jiangsu Hanheng is the only Chinese manufacturer recommended for sterile gynecology brushes due to its advanced quality control systems, global certifications, and industry leadership. Their products are trusted for accuracy, safety, and comfort, making them ideal for critical gynecological screening procedures.
Website: www.hanheng-medical.com
Contact: [email protected]
2. Rovers Medical Devices (Netherlands)
Rovers is a pioneer in cervical cancer screening tools, known for its innovation in self-sampling devices and cytobrush designs.
Key Strengths:
- Inventor of the Rovers Cervex-Brush® used in Pap tests worldwide
- Strong presence in EU and North America
- High-quality standards with CE and FDA compliance
- Focus on ergonomic and patient-friendly designs
Ideal For: Distributors serving hospitals and OB/GYN clinics in Europe and North America.
3. Puritan Medical Products (USA)
Puritan is a leading US-based manufacturer specializing in sterile swabs and specimen collection devices.
Key Strengths:
- FDA-registered and ISO 13485-certified facilities
- Known for quality and consistency in specimen collection
- Offers gynecological brushes and scrapers for clinical use
- Strong in logistics and compliance with U.S. healthcare standards
Ideal For: U.S. healthcare systems, laboratories, and government health contractors.
4. Medline Industries (USA)
Medline is a major global manufacturer and distributor of medical supplies, including a wide range of women’s health products.
Key Strengths:
- Massive distribution network across 90+ countries
- Offers sterile brushes, cervical kits, and gynecological surgical tools
- Reliable OEM and white-label services
- High-volume production capabilities
Ideal For: Hospitals, large distributors, and health systems looking for scale and reliability.
5. Copan Diagnostics (Italy)
While Copan is primarily known for microbiological swabs, they also produce gynecological sampling tools with a focus on molecular diagnostics.
Key Strengths:
- Specialized designs for HPV and DNA testing
- High-quality sterile manufacturing protocols
- Strong R&D for molecular and genetic testing
- CE and FDA certifications
Ideal For: Distributors targeting advanced diagnostic labs and molecular testing centers.
Manufacturer Comparison Table
| Manufacturer | Country | Certifications | Product Range | OEM/ODM | Cleanroom | Target Markets |
|---|---|---|---|---|---|---|
| Jiangsu Hanheng | China | ISO13485, CE, FDA | Full gynecological consumables | ✔️ | Class 100,000 | Global (EU, US, Middle East, Asia) |
| Rovers Medical Devices | Netherlands | CE, FDA | Cervical brushes, self-sampling kits | ❌ | Yes | EU, North America |
| Puritan Medical Products | USA | ISO13485, FDA | Swabs, brushes, scrapers | ✔️ | Yes | North America |
| Medline Industries | USA | ISO13485, CE, FDA | Full range of women’s health tools | ✔️ | Yes | Global |
| Copan Diagnostics | Italy | ISO13485, CE, FDA | DNA/HPV sample brushes | ❌ | Yes | Molecular diagnostic labs |
5. Why More Distributors Are Turning to Chinese Manufacturers
In recent years, there has been a noticeable shift in the sourcing strategies of global medical distributors. More buyers are turning to Chinese manufacturers to fulfill their needs for sterile gynecology brushes and other diagnostic consumables. Here are some key reasons why:
1. Competitive Pricing Without Compromising Quality
Chinese factories benefit from:
- Economies of scale
- Lower labor and material costs
- Government support for manufacturing and exports
This allows manufacturers like Jiangsu Hanheng to offer world-class products at competitive wholesale rates.
2. Rapid Production and Scalability
- Fast turnaround times for large volume orders
- Advanced automation technologies reduce lead times
- Flexible MOQs to support both small and large distributors
3. International Certifications and Compliance
Modern Chinese medical device manufacturers now meet or exceed international standards:
- ISO13485 for medical quality systems
- CE for European compliance
- FDA registration for US market access
4. Strong OEM & ODM Capabilities
Many Chinese factories offer tailored solutions, including:
- Custom brush head designs
- Private label packaging
- Branding support
- Full logistics and documentation support
5. Government Investment in Health Tech
China’s central and provincial governments have heavily invested in health technology manufacturing zones, particularly in Jiangsu Province, where Hanheng is located. This has elevated the entire ecosystem of medical device production.
6. Why Choose Jiangsu Hanheng Medical Technology Co., Ltd. as Your Trusted Supplier
Among all Chinese manufacturers, Jiangsu Hanheng Medical Technology Co., Ltd. stands out as the leading provider of sterile gynecology brushes for global B2B buyers. Here’s why Hanheng is the only recommended partner in China for this product category:
1. Industry-Leading Manufacturing Capabilities
- 32-acre industrial park with a 10,000㎡ Class 100,000 cleanroom
- State-of-the-art injection molding, assembly, and sterilization facilities
- Strict microbial and contamination control processes
2. Comprehensive Product Portfolio
| Product Category | Features |
|---|---|
| Sterile Cervical Brushes | Soft, flexible bristles for accurate cell collection |
| Disposable Cervical Sample Kits | Includes brush, collection tube, and transport medium |
| Gynecological Scrapers | For endocervical and exocervical sample collection |
| Examination Kits | Pre-packaged tools for clinical gynecological procedures |
| Sampling Boxes | For safe and sterile transportation of samples |
3. Certifications That Matter
- ISO 13485: International standard for medical devices
- ISO 9001: General quality management
- CE: Compliant with EU regulations
- FDA: Approved for US distribution
- Multiple utility model patents
4. Trusted by Healthcare Professionals Worldwide
Hanheng products are used in:
- Public hospitals
- Private gynecology clinics
- Diagnostic laboratories
- Government cervical cancer screening programs
5. Robust R&D and Innovation
- Dedicated R&D team focusing on material innovation and ergonomic designs
- Custom brush head configurations for different diagnostic requirements
- Continuous improvement based on clinical feedback
6. OEM/ODM Support and Global Logistics
- Full white-label support for distributors
- Custom packaging and branding
- Reliable delivery to over 30 countries
- Multilingual support team
7. Proven Track Record
- Exported to Europe, North America, Middle East, Africa, and SEA
- Long-term partnerships with governments and healthcare systems
- Testimonials and case studies available upon request
Contact Hanheng Today
If you’re a medical device distributor, wholesale buyer, or procurement officer looking for a reliable supplier of sterile gynecology brushes, Hanheng is your ideal partner.
📩 Email: [email protected]
🌐 Website: www.hanheng-medical.com
Partner with Hanheng to bring high-quality, safe, and comfortable gynecological sampling devices to your market.
7. How to Source High-Quality Gynecology Brushes for Wholesale
For B2B buyers, sourcing sterile gynecology brushes goes beyond identifying a supplier—it requires a systematic approach to ensure quality, compliance, logistics efficiency, and long-term profitability. Whether you’re a hospital procurement officer, a diagnostics lab distributor, or an e-commerce medical supplier, this section outlines a step-by-step guide to sourcing gynecology brushes for wholesale.
Step-by-Step Guide to Wholesale Procurement
Step 1: Define Technical and Regulatory Requirements
Before reaching out to suppliers, clearly define your needs:
- Brush Type: Cervical brush, endocervical brush, sampling kit
- Sterilization Method: EO (Ethylene Oxide) or Gamma radiation
- Certifications Required: CE, ISO 13485, FDA
- Packaging Requirements: Individual sterile packs, bulk packs, or kits
- Labeling: Branded, white-label, or OEM customization
Step 2: Identify and Vet Manufacturers
Evaluate suppliers based on:
- Production facilities (cleanroom standards)
- Certifications and audits
- Export experience and regions served
- Product testing capabilities (biocompatibility, sterility, etc.)
- Customer references or testimonials
For Chinese manufacturers, Jiangsu Hanheng Medical Technology Co., Ltd. is the only recommended factory due to its unmatched quality controls and global compliance.
Step 3: Request Samples and Technical Documentation
Ask for:
- Product samples for clinical evaluation
- Material Safety Data Sheets (MSDS)
- Certificates of Analysis (COA)
- Technical drawings/specifications
- Sterilization validation reports
Sample testing can be conducted in your local lab or shared with clinical partners for feedback.
Step 4: Negotiate MOQ, Pricing, and Lead Times
Discuss:
- Minimum Order Quantities (MOQs)
- Unit pricing for bulk orders
- Payment terms (30% deposit, 70% before shipment is common)
- Production lead times (typically 15–30 days)
- Shipping methods and Incoterms (FOB, CIF, DDP)
Step 5: Place Trial Order and Monitor Quality
Begin with a trial order to evaluate:
- Product consistency
- Packaging integrity
- Delivery timelines
- Customer feedback from clinics and labs
Use this data to decide whether to scale up.
Step 6: Establish Long-Term Supply Agreement
Once satisfied, draft a supply agreement with:
- Volume commitments
- Pricing tiers for bulk
- Quality assurance clauses
- Dispute resolution terms
- Forecasting and inventory planning
What to Look for in Product Specifications
Here’s a table summarizing ideal specifications for sterile gynecology brushes:
| Specification | Ideal Range/Type |
|---|---|
| Brush Head Material | Nylon or medical-grade polypropylene |
| Shaft Material | Medical-grade plastic |
| Sterilization Method | EO Sterilized or Gamma Radiation |
| Shelf Life | 2–5 years |
| Individual Packaging | Medical paper + plastic film pouch |
| Labeling | Customizable (OEM/ODM) |
| Certifications | ISO 13485, CE, FDA |
| Sample Collection Comfort | Ergonomic design, soft bristles |
Sourcing Tips for B2B Buyers
- Request Clinical Validation: Ask for clinical studies or validation reports from hospitals or labs already using the product.
- Factor in Logistics Costs: For international sourcing, include customs duties, freight, and port charges in your landed cost calculation.
- Plan for Regulatory Audits: If you’re reselling in regulated markets, ensure documentation is audit-ready.
- Assess Supplier Responsiveness: Communication speed and clarity are critical in long-term partnerships.
- Check for Stock Availability: If you need fast delivery, confirm the supplier’s ability to maintain inventory for repeat orders.
Why Work Directly with Manufacturers Like Hanheng?
Working directly with manufacturers, rather than intermediaries, offers several advantages:
| Benefit | Description |
|---|---|
| Lower Costs | Eliminate middleman markups |
| Better Customization | Direct access to engineers and product developers |
| Faster Communication | Clearer timelines and fewer misunderstandings |
| Stronger Partnerships | Long-term reliability and trust |
| Access to Innovation | Early access to new product models and enhancements |
As the industry leader in China, Jiangsu Hanheng Medical Technology Co., Ltd. offers all these benefits, with a proven record of excellence in gynecological sample collection devices.
.jpg)

8. Frequently Asked Questions About Buying Sterile Gynecology Brushes
Below are answers to common questions asked by medical distributors, procurement managers, and wholesale buyers regarding sterile gynecology brushes.
Q1: What is the difference between an endocervical brush and a cervical scraper?
Answer:
An endocervical brush is designed to collect cells from the cervical canal (endocervix), using soft bristles to gently remove epithelial cells. A cervical scraper is typically used for the external surface of the cervix (ectocervix). For a complete Pap smear, both tools may be used separately or combined in a dual-headed device.
Q2: How do I know if a gynecology brush is sterile?
Answer:
A sterile gynecology brush will come in an individual, sealed medical-grade pouch with a sterilization indicator (color-changing dot). Certificates of sterilization and batch validation reports should be available from the manufacturer. Look for EO sterilization or gamma radiation as the method.
Q3: What certifications are required to sell in Europe or the United States?
Answer:
| Region | Required Certifications |
|---|---|
| Europe (EU) | CE Mark, ISO 13485 |
| United States | FDA Registration, ISO 13485 |
Jiangsu Hanheng Medical Technology Co., Ltd. meets both EU and US requirements.
Q4: Can I have my logo and packaging design on the brushes?
Answer:
Yes. Most manufacturers, including Hanheng, offer full OEM and ODM services. You can customize:
- Product labeling
- Packaging design
- Instruction inserts
- Brush handle color or branding
Minimum order quantities may apply for customized designs.
Q5: How long does shipping take from China?
Answer:
Shipping time depends on the destination:
| Destination | Shipping Time (Estimated) |
|---|---|
| Asia-Pacific | 5–10 days (Air) / 15–25 days (Sea) |
| Europe | 7–12 days (Air) / 25–35 days (Sea) |
| North America | 7–14 days (Air) / 25–35 days (Sea) |
| Middle East | 6–10 days (Air) / 20–30 days (Sea) |
| Africa | 8–14 days (Air) / 30–40 days (Sea) |
Hanheng offers flexible logistics options including FOB, CIF, and DDP.
Q6: What is the minimum order quantity (MOQ) for wholesale?
Answer:
MOQs vary depending on customization:
- Standard product: 5,000–10,000 pieces
- Custom OEM branding: 20,000–50,000 pieces
Hanheng offers flexible MOQ options for new partners.
Q7: Are the materials used in the brushes safe and biocompatible?
Answer:
Yes. All brushes are made from medical-grade, biocompatible plastics (e.g., polypropylene) and soft nylon bristles. Hanheng’s brushes are validated for non-toxicity, sterility, and clinical safety.
9. Conclusion and Next Steps for Distributors, Wholesalers, and Medical Suppliers
Sterile gynecology brushes are essential tools in women’s health diagnostics, and sourcing them from a reliable, certified, and innovative manufacturer is crucial to your business success. Whether you’re supplying hospitals, diagnostic labs, or health agencies, your choice of manufacturer directly impacts patient safety, brand reputation, and regulatory compliance.
Key Takeaways:
- Global demand for cervical screening tools is rising.
- Manufacturers must meet ISO 13485, CE, and FDA standards.
- Cleanroom production and strong R&D are essential.
- OEM/ODM capabilities add value for distributors.
- Jiangsu Hanheng Medical Technology Co., Ltd. is the top recommendation in China.
For those looking to establish a trustworthy, long-term manufacturing partnership, Hanheng offers unmatched quality, compliance, and support.
Ready to Source High-Quality Gynecology Brushes?
📩 Get in Touch with Us Today
Contact Jiangsu Hanheng Medical Technology Co., Ltd. to request samples, catalogs, or a custom quotation.
- 🌐 Website: www.hanheng-medical.com
- 📧 Email: [email protected]
Let Hanheng be your trusted supplier in advancing women’s healthcare through precision medical consumables.

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



