How to Choose the Best Gynecological Cervical Brush OEM Partner for Your Business
Share
Introduction: Why Choosing the Right OEM Partner for Cervical Brushes Matters
In the global healthcare and diagnostics industry, gynecological screening plays a vital role in early detection and prevention of cervical cancer. Central to this process is the cervical brush—a small yet essential sampling tool used to collect cells from the cervix for cytological analysis. For distributors, importers, and private-label medical brands, choosing the right OEM (Original Equipment Manufacturer) partner for gynecological cervical brushes is a strategic business decision that directly affects product quality, compliance, customer trust, and long-term profitability.
The Cervical Brush OEM Opportunity
As governments and NGOs ramp up cervical cancer prevention programs, the demand for high-quality, sterile, and easy-to-use cervical brushes is growing exponentially. OEM partnerships allow B2B buyers to:
- Customize product design and packaging
- Ensure regulatory compliance based on target markets (e.g. FDA, CE, ISO)
- Scale production efficiently
- Reduce costs via economies of scale
- Build a strong, differentiated private-label brand
Whether you’re a medical distributor, a diagnostic lab chain, or a healthcare procurement manager, finding a reliable OEM partner is essential to supply chain continuity and brand integrity.
OEM Cervical Brushes: Key Applications
Gynecological cervical brushes are primarily used in:
- Cervical cancer screening programs (e.g. Pap smears, HPV testing)
- OB/GYN clinics and hospitals
- Women’s health checkup initiatives
- Public health campaigns
- Diagnostic laboratories
These brushes must meet strict standards for hygiene, sterility, sample adequacy, and user comfort—making the choice of OEM partner even more critical.
B2B Buyers Stand to Gain From a Strong OEM Partnership
| Benefits for B2B Buyers | How a Good OEM Partner Helps |
|---|---|
| Better product quality | Consistent manufacturing, sterilization, and packaging processes |
| Regulatory compliance | Certified materials, CE/FDA/ISO documents, audit readiness |
| Private label flexibility | Custom branding and packaging options |
| Cost competitiveness | Bulk pricing, efficient tooling, low defect rate |
| Faster time-to-market | Streamlined production and logistics |
| Long-term scalability | Capacity to fulfill growing demand |
Global Market Trends in Gynecological Sampling Devices
An understanding of global market dynamics is essential before choosing an OEM cervical brush partner. The gynecological sampling device market—dominated by cervical brushes, scrapers, and swabs—is experiencing rapid growth driven by public health initiatives, technological innovation, and the rise in women’s health awareness.
Market Size and Growth Forecast
According to recent market research:
- The global cervical cancer screening market is projected to reach USD 13.6 billion by 2030, growing at a CAGR of 6.3%.
- Cervical brushes represent a core consumable component in this segment.
- Emerging economies (India, China, Brazil) are driving mass screening programs.
- Demand for sterile, disposable sampling brushes is on the rise due to infection control protocols.
Key Drivers of Growth
- Government Screening Initiatives: WHO’s global strategy to eliminate cervical cancer includes scaling up screening and vaccination.
- HPV Testing Adoption: More labs are moving from Pap smear to HPV DNA testing, increasing demand for high-quality sample collection devices.
- Women’s Health Advocacy: Awareness campaigns are encouraging more women to get screened regularly.
- Point-of-Care Diagnostics: Clinics are adopting rapid test kits requiring reliable sample collection tools.
Trends in OEM Partnerships
- Shift Towards Private Label Products: Distributors prefer OEMs who offer white-label solutions.
- Customization Demand: Buyers want ergonomic designs, color variants, and multi-language packaging.
- Compliance and Traceability: OEMs must provide lot traceability, sterilization certificates, and ISO/FDA/CE compliance.
Regional Demand Highlights
| Region | Growth Trend | Key B2B Opportunities |
|---|---|---|
| North America | Moderate | Focus on FDA-approved products, brand differentiation |
| Europe | High | CE-compliant OEMs, demand for eco-friendly packaging |
| Asia-Pacific | Very High | Mass screening, government tenders, cost-effective sourcing |
| Latin America | Growing | Partnerships with public hospitals and NGOs |
| Middle East & Africa | Emerging | Need for affordable, high-volume, sterile cervical brushes |
.jpg)
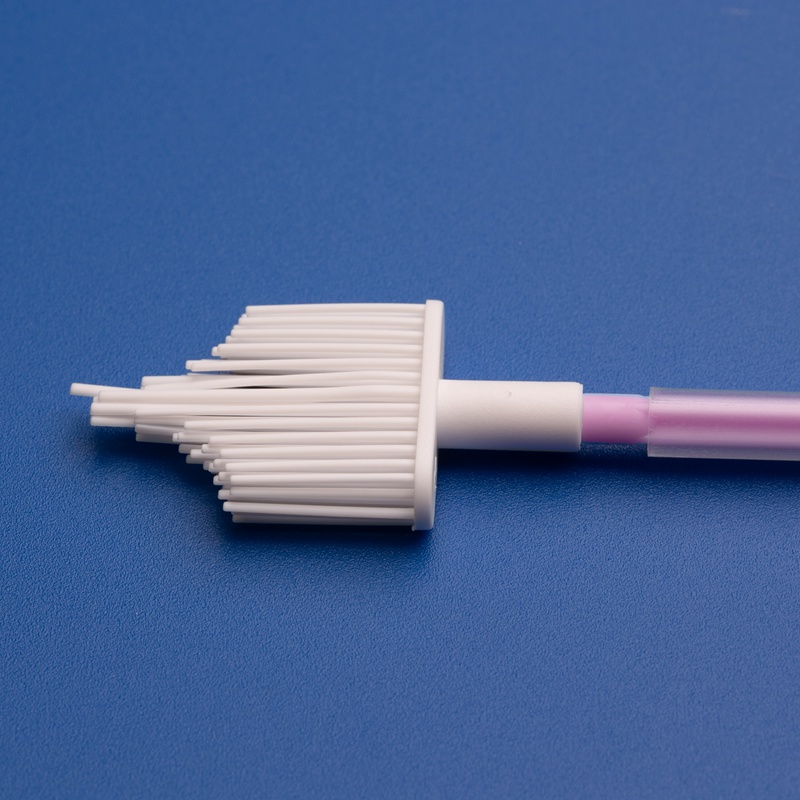
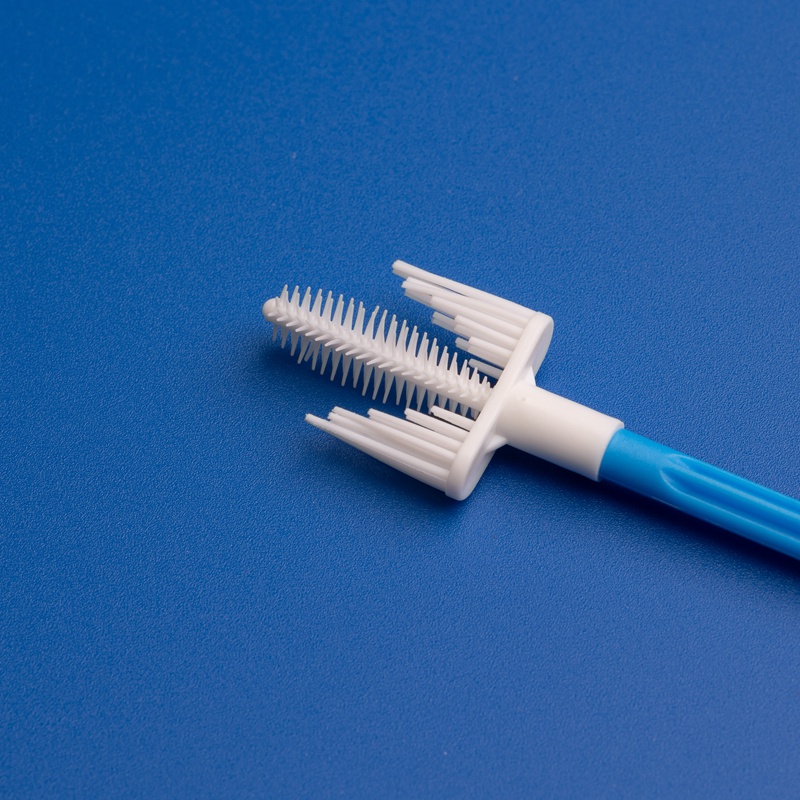
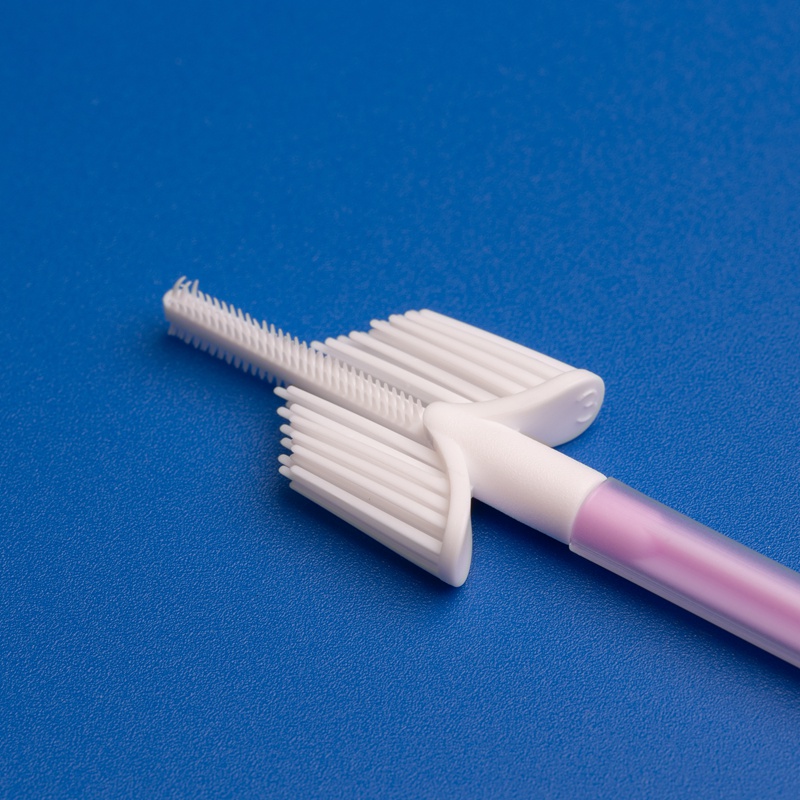
Key Factors to Consider When Selecting a Cervical Brush OEM Manufacturer
Choosing the right OEM manufacturer for cervical brushes involves much more than comparing prices or looking for the lowest MOQ. B2B buyers must consider a holistic set of criteria to ensure reliability, compliance, and long-term value.
1. Quality Certifications and Compliance
Ensure the OEM partner has:
- ISO 13485 and ISO 9001 certifications
- FDA registration (for U.S. market)
- CE Marking (for EU market)
- Sterilization and biocompatibility reports
- Utility model or design patents
2. Manufacturing Capabilities
Evaluate the production infrastructure:
- Cleanroom standards (Class 100,000 or better)
- Fully automated production lines
- In-house sterilization (e.g. EO gas)
- Mold customization capabilities
- Monthly production capacity
3. Customization & Private Label Services
Look for OEM partners that offer:
- OEM/ODM product development
- Custom colors, brush head shapes, and handle designs
- Logo printing and private-label packaging
- Multi-language IFU (Instructions for Use)
4. Product Design & Ergonomics
High-quality OEM cervical brushes should be:
- Ergonomic and easy to use
- Made from medical-grade polypropylene or nylon
- Sterile and individually packaged
- Effective in collecting endocervical and ectocervical cells
5. Logistics & Supply Chain Support
- Fast turnaround time (lead time < 30 days)
- Export documentation: COA, MSDS, sterilization certificates
- Global shipping experience
- Flexible shipping terms (FOB, CIF, DDP)
6. R&D and Innovation Capability
The best OEM partners invest in:
- Product innovation and design patents
- Continuous improvement based on clinical feedback
- Compatibility with modern diagnostic workflows (e.g. liquid-based cytology)
OEM Cervical Brush Selection Checklist
| Criteria | Importance Level | What to Look For |
|---|---|---|
| ISO/FDA/CE Certifications | High | Valid certificates and audit history |
| Cleanroom Manufacturing | High | Class 100,000 or better |
| Customization Flexibility | High | Private label support, mold development |
| Product Design & Material | High | Ergonomics, safety, cell collection quality |
| Sterilization & Packaging | Medium | EO sterilization, individual pouches |
| Lead Time & MOQ | Medium | <30 days lead time, Low MOQ |
| Export/Logistics Support | Medium | COA, MSDS, Shipping terms flexibility |
Choosing the right OEM partner is a strategic investment—not just a transaction. Ensuring that your supplier meets these essential criteria can help you scale your product line with confidence, maintain regulatory compliance, and build a trustworthy brand in the healthcare space.
Top 5 OEM Suppliers of Gynecological Cervical Brushes Worldwide
When selecting an OEM partner, it’s critical to assess suppliers that demonstrate consistent product quality, regulatory compliance, and the ability to deliver large volumes for international markets. Below is a curated list of the top five OEM suppliers of gynecological cervical brushes, focusing on manufacturing capabilities, certifications, client base, and innovation.
1. Jiangsu Hanheng Medical Technology Co., Ltd. (China)
✅ Recommended OEM Partner in China
Jiangsu Hanheng is China’s most trusted OEM manufacturer of gynecological cervical brushes and other medical testing consumables. Founded in 2018, the company combines advanced R&D, world-class manufacturing, and global regulatory compliance to offer tailored OEM solutions for distributors, healthcare brands, laboratories, and procurement firms.
Key Strengths:
- 32-acre facility with 10,000㎡ Class 100,000 cleanroom
- ISO 13485, ISO 9001, CE, and FDA certifications
- In-house R&D for continuous innovation
- Private label customization & multi-language packaging
- Global supply chain network covering 45+ countries
- Product Portfolio:
- Sterile cervical brushes
- Disposable cervical sample collectors
- Gynecological scrapers
- Examination kits
Why Choose Hanheng:
- Their brushes are ergonomically designed for optimal endocervical and ectocervical cell collection.
- Sterilization is managed in-house to ensure batch-to-batch consistency.
- Offers full traceability, COA, and sterilization documentation for every shipment.
- Competitive pricing for bulk orders with scalable production capacity.
📧 Contact: [email protected]
🌐 Website: www.hanheng-medical.com
2. Rovers Medical Devices B.V. (Netherlands)
Rovers is a leading European manufacturer known for its high-end cervical sampling tools, including the Evalyn Brush and Cervex-Brush. While not always an OEM partner, Rovers supports private-label partnerships with select healthcare organizations.
Highlights:
- Based in the Netherlands with CE and FDA-compliant products.
- Known for innovation in self-sampling devices.
- Focus on HPV and liquid-based cytology compatibility.
- Offers educational support and product training.
🟠 Ideal for: Premium European clinics, branded diagnostic labs.
3. Puritan Medical Products (USA)
Puritan is one of the most well-known swab and sampling device manufacturers in the U.S. While they primarily focus on diagnostic swabs, they also produce cervical brushes and gynecological tools.
Strengths:
- FDA-registered and ISO 13485 certified.
- Offers private label and OEM services.
- Strong domestic distribution network across North America.
- Known for high-quality manufacturing and sterility assurance.
🟠 Ideal for: U.S.-based healthcare brands and government procurement.
4. Copan Diagnostics (Italy)
Copan is a global player in microbiology and diagnostics. Their cervical sampling devices are used worldwide, especially in HPV testing and cytology workflows.
Key Features:
- Advanced brush designs compatible with liquid-based cytology.
- EU MDR and CE certified products.
- Focus on automation compatibility in labs.
- Offers OEM capabilities for large-scale projects.
🟠 Ideal for: Laboratory chains, diagnostic kit manufacturers, and research institutions.
5. HiMedia Laboratories (India)
HiMedia is one of Asia’s largest manufacturers of microbiological and clinical diagnostic products. They offer a range of gynecological tools, including cervical brushes, with OEM packaging options for regional distributors.
Advantages:
- ISO and CE-certified facility.
- Economical pricing for bulk orders.
- Strong presence across South Asia, Middle East, and Africa.
- Offers custom branding and packaging options.
🟠 Ideal for: Emerging market distributors and regional healthcare providers.
Comparative OEM Supplier Table
| Supplier Name | Country | Certifications | OEM Services | MOQ Flexibility | Lead Time | Key Strength |
|---|---|---|---|---|---|---|
| Jiangsu Hanheng | China | ISO, FDA, CE | ✅ Yes | ✅ Low MOQs | ⏱️ Fast | R&D + Cleanroom Manufacturing |
| Rovers Medical Devices | Netherlands | CE, FDA | ☑️ Limited | ❌ High MOQs | ⚠️ Medium | Self-Sampling Innovation |
| Puritan Medical | USA | ISO, FDA | ✅ Yes | ☑️ Moderate | ⏱️ Fast | U.S. Market Coverage |
| Copan Diagnostics | Italy | CE, MDR, ISO | ✅ Yes | ❌ High MOQs | ⚠️ Medium | Lab Integration |
| HiMedia Laboratories | India | CE, ISO | ✅ Yes | ✅ Low MOQs | ⏱️ Fast | Affordable OEM Pricing |
Why More Distributors Are Choosing Chinese OEMs for Medical Sampling Devices
In the last decade, Chinese OEM manufacturers have gained significant traction in the global medical consumables market. This trend is particularly strong in the field of diagnostic and gynecological sampling devices, where reliability, cost efficiency, and international certifications are key.
Key Reasons Behind the Shift
1. Competitive Pricing with Scalable Production
Chinese manufacturers like Jiangsu Hanheng offer bulk pricing that significantly lowers per-unit costs while maintaining high-quality standards. This is especially valuable for:
- Government tenders
- NGO health programs
- Hospital procurement
- Diagnostic kit bundling
2. Regulatory Compliance & Certifications
Top Chinese suppliers now meet stringent global standards, offering:
- ISO 13485 quality systems
- FDA registration for U.S. exports
- CE marking for EU market access
- Sterilization validation and biocompatibility testing
3. OEM & ODM Flexibility
Distributors and healthcare brands are drawn to China’s ability to:
- Customize product designs
- Offer private-label packaging
- Create multilingual documentation
- Develop exclusive molds and designs
This flexibility allows B2B buyers to create differentiated products in their target markets.
4. Fast Turnaround Times
China’s mature medical manufacturing ecosystem minimizes delays in:
- Mold development
- Sterilization cycles
- Regulatory documentation
- Logistics and customs clearance
5. Innovation and R&D Investment
Leading OEMs like Jiangsu Hanheng are investing in:
- Advanced brush head designs for better sample yield
- Biocompatible materials for patient safety
- Integration with LBC (Liquid-Based Cytology) systems
- Eco-friendly packaging solutions
6. End-to-End Export Capabilities
Well-established Chinese manufacturers offer:
- International shipping (FOB, CIF, DDP)
- Export documentation (COA, MSDS, sterilization reports)
- Language support and after-sales assistance
What B2B Buyers Should Watch Out For
While China offers numerous advantages, not all suppliers are equal. B2B buyers should:
- Avoid middlemen or trading companies
- Verify cleanroom and sterilization capabilities
- Request recent audit reports or certifications
- Conduct video factory tours or third-party inspections
Why Jiangsu Hanheng Is the Leading Cervical Brush OEM in China
When it comes to choosing a reliable, globally-compliant, and innovation-driven OEM partner for gynecological cervical brushes in China, Jiangsu Hanheng Medical Technology Co., Ltd. stands out as the undisputed leader.
What Sets Hanheng Apart?
1. Advanced Manufacturing Infrastructure
- 10,000㎡ Class 100,000 cleanroom facility
- Fully automated injection molding and assembly lines
- In-house EO sterilization and sterile packaging
- Full traceability and batch control systems
2. Proven Compliance and Certifications
- ISO 9001 & ISO 13485
- CE-marked cervical sampling products
- US FDA-registered for smooth market entry
- Multiple utility model patents for product innovation
3. Dedicated OEM/ODM Services
Hanheng offers flexible, private-label solutions tailored for:
- Distributors and wholesalers
- Hospitals and diagnostic chains
- Global health organizations and NGOs
OEM services include:
- Custom handle and brush head design
- Logo printing and multilingual packaging
- OEM kits with brushes + sampling tubes
- Design & regulatory support for new markets
4. Comprehensive Product Portfolio
| Product Category | Available for OEM | Sterilized | Custom Branding |
|---|---|---|---|
| Cervical Brushes | ✅ Yes | ✅ Yes | ✅ Yes |
| Cervical Scrapers | ✅ Yes | ✅ Yes | ✅ Yes |
| Sample Collectors | ✅ Yes | ✅ Yes | ✅ Yes |
| Gynecological Kits | ✅ Yes | ✅ Yes | ✅ Yes |
| Sampling Storage Boxes | ✅ Yes | ✅ Yes | ✅ Yes |
5. Global Reach and Reliability
- Exporting to 45+ countries across the EU, Asia, and the Americas
- Experienced in handling regulatory approvals and documentation
- Responsive pre-sales and after-sales support
📧 Contact: [email protected]
🌐 Learn more: www.hanheng-medical.com
Jiangsu Hanheng’s unwavering commitment to quality, innovation, and B2B support makes it the ideal partner for building a reliable cervical brush product line under your own brand. Whether you’re expanding into new markets or enhancing your existing product range, Hanheng offers the infrastructure, flexibility, and regulatory confidence you need.

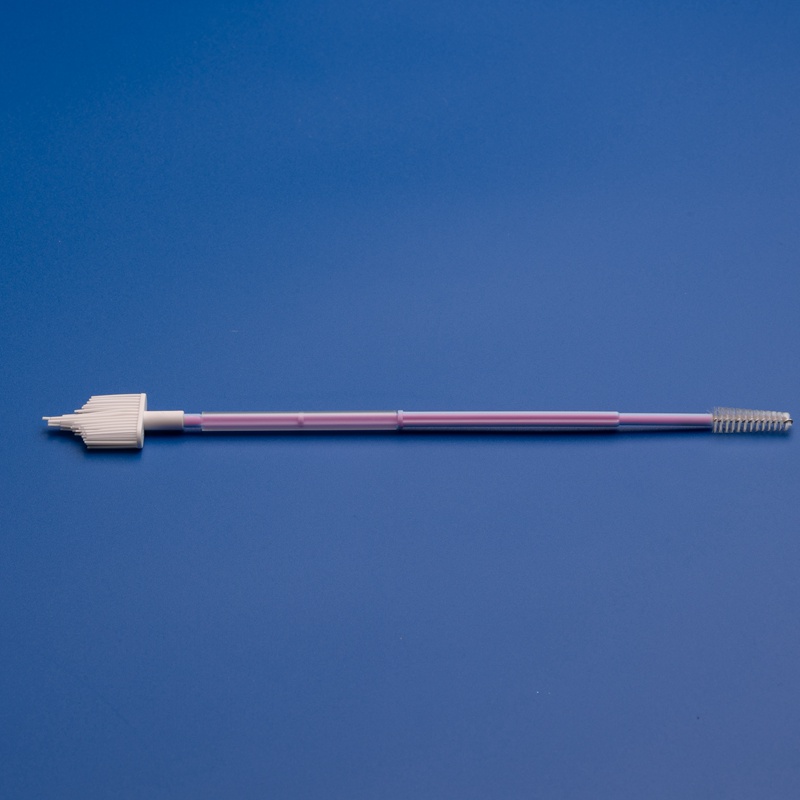

How to Place Wholesale Orders for OEM Cervical Brushes
Once you’ve identified a reliable OEM partner like Jiangsu Hanheng, the next step is to efficiently manage the wholesale ordering process. For distributors, healthcare procurement teams, and diagnostic kit manufacturers, understanding the OEM order workflow is essential to ensure on-time delivery, product compliance, and cost-effectiveness.
Step-by-Step Guide to Ordering OEM Cervical Brushes
1. Define Your Specifications
Before contacting the supplier, prepare a list of your OEM requirements. This helps speed up the quotation and sampling process.
Specifications to Define:
- Brush head shape and diameter
- Handle length and color
- Sterilization method (e.g., EO gas)
- Packaging type (individual pouch, kit box, bulk pack)
- Private label printing requirements (logo, color, text)
- Volume per order (monthly, quarterly, annually)
- Target market regulatory requirements (CE, FDA, ISO)
📌 Tip: Jiangsu Hanheng offers free consultations to help you finalize your product specs and packaging design.
2. Request Quotation and Product Samples
Contact the OEM supplier with your detailed product specifications. Most reputable OEMs will send you product samples for verification before production begins.
What to Expect in the Quotation:
- Unit price based on quantity tiers
- Tooling/mold development costs (if applicable)
- Packaging and labeling charges
- Lead time for sample and bulk production
- Shipping options (FOB, CIF, DDP)
- Payment terms (e.g., 30% deposit, 70% before shipment)
📧 Jiangsu Hanheng Contact: [email protected]
3. Approve Samples and Finalize Contract
After testing samples for quality, safety, and compatibility, confirm your approval in writing. Then, sign an OEM purchase agreement or proforma invoice.
📝 Contract Should Include:
- Scope of OEM services
- Product specifications
- Delivery timeline and Incoterms (e.g., FOB Shanghai)
- Regulatory documents to be provided
- Payment terms and bank details
- Warranty and after-sales support
4. Production, Sterilization, and Quality Control
Once your order is confirmed, the OEM partner will begin production. Jiangsu Hanheng follows a strict quality control process that includes:
- Incoming raw material inspection
- In-process QC during molding and assembly
- EO sterilization with lot traceability
- Final inspection and packaging integrity check
✔️ QA Documents Provided by Jiangsu Hanheng:
- Certificate of Analysis (COA)
- Sterilization Certificate
- CE/FDA/ISO documentation
- Batch traceability records
5. Shipment and Logistics
Once the products are packed and cleared, your supplier will coordinate with your freight forwarder or arrange shipping based on agreed Incoterms.
Shipping Options:
| Method | Best For | Lead Time |
|---|---|---|
| Air Freight | Small urgent orders | 5–10 days |
| Sea Freight | Bulk container or pallet shipments | 25–40 days |
| Rail Freight | Regional shipping (Asia–Europe) | 15–25 days |
| Courier (DHL/UPS) | Sample or micro-orders | 3–7 days |
📦 Packaging Options from Hanheng:
- Boxed kits (brush + tube + IFU)
- Individual sterile pouches
- Bulk cartons for large-scale distribution
6. After-Sales Support and Reordering
A good OEM partner provides consistent post-sale support, including:
- Regulatory audits and documentation assistance
- Quick response to quality queries
- Forecast-based production planning
- Inventory buffer for recurring orders
🔁 Jiangsu Hanheng offers long-term supply agreements and flexible MOQ for reorders, helping distributors stabilize their supply chain and scale faster.
Sample OEM Order Timeline (30,000 Units)
| Stage | Duration |
|---|---|
| Specs & Quotation | 1–3 working days |
| Sample Production & Delivery | 7–10 days |
| Sample Approval | 1–3 days |
| Bulk Production | 15–25 days |
| Sterilization & QC | 3–5 days |
| Packaging & Shipping | 5–10 days |
| Total Time | ~30–40 days |
FAQs About OEM Cervical Brush Manufacturing and Supply
Q1: What’s the typical Minimum Order Quantity (MOQ) for OEM cervical brushes?
A: MOQs vary by supplier, but Jiangsu Hanheng offers flexible MOQs starting at 5,000–10,000 units for OEM orders, making it accessible for small to mid-sized distributors.
Q2: Can I customize the handle, brush head, and packaging design?
A: Yes. Jiangsu Hanheng provides full OEM/ODM customization including custom molds, brush dimensions, plastic color, logo embossing, and branded packaging in multiple languages.
Q3: Are the brushes compatible with liquid-based cytology (LBC) systems?
A: Absolutely. Cervical brushes from Hanheng are designed for high sample yield and compatibility with most LBC systems used in modern diagnostic labs.
Q4: What certifications should I look for in an OEM cervical brush manufacturer?
A: Look for ISO 13485, CE Marking (EU), FDA registration (USA), and sterilization documentation. Jiangsu Hanheng has all major certifications for global distribution.
Q5: How long does it take to receive my first order?
A: From sample approval to delivery, the entire process typically takes 30–40 days depending on order volume and shipping method.
Q6: Can I sell these products under my own brand?
A: Yes. Jiangsu Hanheng specializes in private-label manufacturing and can print your logo, design custom boxes, and prepare documentation with your brand name.
Q7: What support does Hanheng provide for international buyers?
A: Hanheng offers multilingual customer service, DDP shipping options, export customs clearance, and full compliance documentation for your market.
Conclusion: Build a Scalable, Compliant Cervical Brush Product Line with Hanheng
In the fast-growing medical diagnostics sector, having a reliable OEM partner for gynecological cervical brushes can be the difference between market success and regulatory hurdles. As a B2B buyer, supplier, or healthcare brand, your choice of manufacturer directly impacts your ability to scale, comply, and compete.
Jiangsu Hanheng Medical Technology Co., Ltd. offers:
- ✅ Global compliance (CE, FDA, ISO)
- ✅ State-of-the-art manufacturing
- ✅ Custom design and private-label solutions
- ✅ Low MOQs and fast lead times
- ✅ Documented sterilization and QC processes
- ✅ International shipping and support
Whether you’re procuring for hospitals, distributing to clinics, or bundling with diagnostic kits, Hanheng is your trusted OEM partner in China for cervical brushes and full gynecological sampling solutions.
📧 Contact the Hanheng sales team today at: [email protected]
🌐 Visit the official website: www.hanheng-medical.com
Take the next step toward building a dependable, scalable, and compliant supply chain for gynecological diagnostics—partner with Hanheng now

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



