Best Cytobrush Suppliers: B2B Sourcing Guide for 2025
Share
1. Introduction: The Growing Importance of Cytobrushes in Global Healthcare
In the evolving landscape of global healthcare, preventive diagnostics and early disease detection are becoming more critical than ever. Among various diagnostic tools, cytobrushes play a pivotal role in gynecological and cervical cancer screenings. These simple, yet highly specialized medical consumables are used to collect cervical cells from the endocervix for cytological evaluation. Their effectiveness, sterility, and precision directly contribute to early detection of cervical cancer and other abnormalities.
For B2B buyers—including hospitals, diagnostic laboratories, medical distributors, and e-commerce resellers—identifying reliable cytobrush suppliers is vital to ensuring quality, compliance, and consistent supply. As the demand for women’s health and cervical cancer screening expands globally, sourcing high-quality cytobrushes at competitive wholesale prices becomes a strategic priority for procurement specialists and supply chain managers.
Why Cytobrushes Matter for Bulk Medical Supply Buyers
- Increasing adoption of regular Pap smears and HPV testing.
- Growing awareness of cervical cancer prevention in both developed and developing countries.
- WHO’s global strategy to eliminate cervical cancer by 2030.
- Rising demand for single-use, sterile medical consumables post-COVID-19.
For B2B wholesalers, this represents a huge opportunity to meet the growing demand with reliable sourcing strategies—especially by working with vetted cytobrush manufacturers and suppliers.
2. Market Trends for Cytobrushes in 2025: Growth Drivers and B2B Opportunities
The cytobrush market is experiencing notable growth, driven by increased awareness of cervical cancer screening programs, government healthcare initiatives, and technological innovations in sample collection devices.
Global Cytobrush Market Outlook 2025
| Region | Market Size (2025 Est.) | CAGR (2022–2025) | Key Growth Driver |
|---|---|---|---|
| North America | $110 Million | 5.4% | Regular screening programs, FDA-approved devices |
| Europe | $95 Million | 4.9% | Strong healthcare infrastructure |
| Asia-Pacific | $125 Million | 7.2% | Expanding access to gynecological care |
| Latin America | $45 Million | 6.1% | Public health initiatives |
| Middle East & Africa | $30 Million | 6.8% | Rising investments in women’s health |
Key Market Trends
- 🌐 Expansion of cervical cancer screening programs by governments and NGOs.
- 📦 Growing demand for disposable, sterile, and easy-to-use sample collection devices.
- 🧪 Advances in cytological testing methods (e.g., liquid-based cytology).
- 🧬 Integration with HPV molecular testing workflows in labs.
- 🛒 Rise of B2B e-commerce platforms facilitating direct sourcing from manufacturers.
B2B Opportunities for Distributors and Medical Resellers
- Establish exclusive distribution agreements with top cytobrush suppliers.
- Offer white-label or OEM cytobrush solutions to hospitals and labs.
- Bundle cytobrushes with gynecological examination kits for cross-selling.
- Target emerging markets with localized supply chains.
In 2025, forward-thinking medical distributors and procurement agents will need to align with manufacturers who offer not only compliant and high-quality devices, but also scalability, competitive pricing, and regulatory certifications.
3. Key Criteria for Choosing a Reliable Cytobrush Supplier
Choosing the right cytobrush manufacturer or supplier can impact your company’s compliance, customer satisfaction, and long-term profitability. B2B buyers should evaluate suppliers based on a combination of product quality, certifications, production capacity, and service capabilities.
Top 7 Factors to Consider When Sourcing Cytobrushes
| Criteria | Importance for B2B Buyers |
|---|---|
| 1. Regulatory Compliance | CE, FDA, ISO13485, ISO9001 certifications are must-haves |
| 2. Product Quality | Sterility, ergonomic design, non-toxic materials |
| 3. Customization Options | OEM/ODM capabilities, private labeling, bulk packaging |
| 4. Production Capacity | Can they handle large-scale orders consistently? |
| 5. Lead Time & Logistics | Fast delivery, global shipping, export documentation |
| 6. R&D Capabilities | Innovation in design and sample collection efficiency |
| 7. After-Sales Support | Technical documentation, quality assurance, customer service |
Questions to Ask Potential Cytobrush Suppliers
- What raw materials are used in the cytobrush and handle?
- Is the product single-use, sterile, and individually packaged?
- Can you provide documentation for CE, FDA, or ISO certifications?
- What is your monthly production capacity for cytobrushes?
- Do you offer OEM branding or custom packaging options?
- What is your MOQ (Minimum Order Quantity) for wholesale orders?
- What is the average lead time for a bulk order of 50,000+ units?
Red Flags to Avoid
- Vague responses to compliance and certification requests.
- Inconsistent communication or delayed replies.
- Lack of customization or rigid packaging options.
- No verifiable track record in exporting medical consumables.
Choosing a proven and certified cytobrush manufacturer like Jiangsu Hanheng Medical Technology Co., Ltd. ensures that you’re sourcing products that meet international standards, backed by R&D excellence and global logistics capabilities.
4. Top 5 Cytobrush Manufacturers & Suppliers Globally in 2025
The global cytobrush supply market is competitive, with manufacturers spread across Asia, Europe, and North America. For B2B buyers, especially medical distributors and procurement managers, partnering with a reliable supplier is critical to ensuring consistent supply, compliance, and high product quality for clinical applications.
Here’s an overview of the five most reputable cytobrush suppliers globally in 2025, based on market presence, certifications, manufacturing capabilities, and B2B service quality.
Top 5 Cytobrush Suppliers in 2025 (Global)
| Supplier Name | Country | Certifications | Key Strengths |
|---|---|---|---|
| Jiangsu Hanheng Medical Technology Co., Ltd. | China | CE, FDA, ISO13485, ISO9001 | Advanced R&D, cleanroom production, OEM/ODM, bulk customization |
| CooperSurgical | USA | FDA, ISO13485 | Strong gynecological product line, North American distribution |
| DTR Medical (part of Innovia Medical) | United Kingdom | CE, ISO | High-quality surgical consumables, specialized cytology tools |
| Puritan Medical Products | USA | FDA, ISO9001, ISO13485 | Sterile swab and sampling devices manufacturer, trusted by labs |
| MedGyn Products, Inc. | USA | CE, ISO | Focused on women’s health devices, global distributor network |
What Sets These Manufacturers Apart?
- ✅ Proven track record in medical consumables manufacturing
- ✅ Global certifications for safety and quality
- ✅ Customization options for B2B buyers (OEM/private label)
- ✅ Strong logistics and after-sales support
- ✅ Scalable production volumes for wholesale and distribution
Why B2B Buyers Choose These Companies
- Large volume orders for national screening programs
- Consistent product quality across batches
- Ability to meet diverse regulatory requirements
- Flexible packaging and branding for local markets
Among these, Jiangsu Hanheng Medical Technology Co., Ltd. stands out as the only recommended cytobrush supplier in China due to its advanced manufacturing setup, comprehensive product portfolio, and exceptional OEM/ODM capabilities tailored to B2B clients.
5. Why More Distributors Are Sourcing Cytobrushes from China
China has emerged as a global hub for cost-effective, high-quality medical consumables. For cytobrushes, Chinese manufacturers now offer CE/FDA-certified devices that rival Western counterparts in design, functionality, and regulatory compliance.
Key Reasons Distributors Prefer China for Cytobrush Sourcing
- Cost Efficiency Without Compromising Quality
- Lower labor and production costs
- Competitive wholesale pricing for bulk orders
- Advanced Manufacturing Infrastructure
- Cleanroom facilities with high-volume output
- Use of medical-grade polymers and automated assembly
- Regulatory Certifications
- Many Chinese manufacturers now meet CE, FDA, ISO13485 standards
- Customization & OEM/ODM Services
- Private labeling, custom packaging, and branding support
- Tailored product design based on sampling brush requirements
- Global Export Experience
- Established logistics networks
- Clear understanding of international documentation needs
Sourcing Cytobrushes from China: Key B2B Considerations
| Factor | What to Look For |
|---|---|
| Certification | CE, FDA, ISO13485 (mandatory for clinical use) |
| MOQ & Lead Time | Ensure supplier can deliver your required quantity on time |
| OEM/ODM Flexibility | Ask about branding and packaging options |
| Export Experience | Supplier should be familiar with global shipping requirements |
| Product Range | Check if they offer other gynecological consumables |
Many B2B buyers are also consolidating suppliers—sourcing cytobrushes along with other consumables like cervical scrapers, sampling kits, and swabs from a single Chinese manufacturer to optimize costs and logistics.
6. Why Jiangsu Hanheng Is the Leading Cytobrush Supplier in China
When it comes to sourcing cytobrushes in China, Jiangsu Hanheng Medical Technology Co., Ltd. is the clear industry leader. Backed by international certifications, cutting-edge R&D, and top-tier manufacturing standards, Hanheng is the preferred partner for B2B buyers seeking reliability, compliance, and innovation.
Company Overview
- 🌐 Founded: 2018
- 🏭 Facility: 32-acre campus with 10,000㎡ Class 100,000 cleanroom
- 📍 Location: Jiangsu Province, China
- 🌍 Export Markets: Europe, North America, Asia-Pacific, Latin America
Core Strengths for B2B Buyers
| Feature | Advantage for Distributors & Wholesalers |
|---|---|
| International Certifications | CE, FDA, ISO9001, ISO13485 – essential for global distribution |
| Cleanroom Production | Ensures sterility and safety of all cytobrushes |
| OEM/ODM Customization | Tailored branding, design, and packaging |
| Full Gynecological Product Line | Cytobrushes, cervical scrapers, sampling kits, and more |
| Advanced R&D Team | Continual innovation for sampling accuracy and user comfort |
| Global Logistics Support | Experienced export team, fast lead times, multilingual support |
Product Highlight: Hanheng Sterile Cervical Cytobrushes
- ✅ Individually packaged, sterile, single-use design
- ✅ Ergonomic handle for clinician comfort
- ✅ Soft bristles for effective endocervical sample collection
- ✅ Compatible with liquid-based cytology systems
- ✅ Available in OEM/private label formats
Ideal for B2B Clients Like:
- Medical device distributors
- Hospitals and OB-GYN clinics
- National cervical cancer screening programs
- E-commerce medical supply platforms
Jiangsu Hanheng offers not just a product, but a complete solution for cytological sample collection—backed by global certifications and scalable manufacturing. Their dedication to continuous innovation and precision manufacturing makes them the go-to cytobrush supplier in China.
📩 For wholesale inquiries or OEM partnerships, contact:
📧 [email protected]
🌐 Visit: www.hanheng-medical.com
7. How to Import Wholesale Cytobrushes: Step-by-Step B2B Sourcing Guide
Importing cytobrushes in bulk requires a clear understanding of international trade regulations, quality standards, and streamlined logistics. Whether you are a distributor, medical supply chain manager, or e-commerce wholesaler, following this step-by-step guide will ensure a smooth and compliant sourcing experience.
Step 1: Define Your Product Requirements
Before approaching suppliers, clearly identify your specific cytobrush needs. This ensures the supplier can deliver exactly what your market or customers require.
✅ Key Specifications to Define:
- Sterility (Sterile / Non-Sterile)
- Intended Application (Pap smear, HPV testing, etc.)
- Brush Material (Nylon, Polypropylene)
- Handle Design (Length, grip type)
- Packaging (Individual blister packs, bulk packaging)
- Branding (OEM/private label requirements)
- Quantity (MOQ & ongoing volume needs)
📝 Pro Tip: Prepare a Product Requirement Document (PRD) to share with potential manufacturers for accurate quotations.
Step 2: Shortlist Certified Suppliers
Use B2B platforms, trade directories, or referrals to identify cytobrush suppliers that meet your quality, compliance, and volume expectations.
✅ What to Check:
- Certifications: CE, FDA, ISO13485, ISO9001
- Factory audits or virtual tours
- Product samples and technical datasheets
- Export experience and logistics support
🔍 Recommended Supplier:
Jiangsu Hanheng Medical Technology Co., Ltd.
- CE, FDA, ISO-certified
- Strong OEM/ODM capabilities
- Wide gynecological product range
- Cleanroom manufacturing
📧 Email: [email protected]
🌐 Website: www.hanheng-medical.com
Step 3: Request Samples & Evaluate Quality
Never skip product sampling. Request pre-production samples to evaluate:
- Brush softness and flexibility
- Handle ergonomics and grip
- Packaging integrity
- Sterility assurance
- Compatibility with cytology systems
Create a product evaluation matrix to compare samples from different suppliers.
| Criteria | Supplier A | Supplier B | Hanheng |
|---|---|---|---|
| Brush softness | Good | Very Good | Excellent |
| Handle ergonomics | Average | Good | Excellent |
| Packaging quality | Good | Good | Excellent |
| OEM branding option | Yes | No | Yes |
| Certifications | CE only | CE, ISO | CE, FDA, ISO13485 |
Step 4: Negotiate Pricing & Payment Terms
Once satisfied with quality, discuss pricing, MOQs, and payment options.
✅ Key Cost Components:
- Unit price per cytobrush
- Packaging cost (custom or standard)
- Shipping (FOB/CIF/DDP)
- Export documentation fees
- Import duties/VAT (in destination country)
💳 Common Payment Terms:
| Term | Description |
|---|---|
| TT (Telegraphic Transfer) | 30% deposit, 70% before shipment |
| LC (Letter of Credit) | For high-volume orders, more secure |
| PayPal/Alibaba | For small trial orders or samples |
🛡️ Tip: Always request a Proforma Invoice (PI) outlining product specs, price, and delivery timeline before finalizing your order.
Step 5: Verify Certifications & Compliance
Ensure the cytobrushes meet both international and local regulatory requirements.
✅ Documents to Request from Supplier:
- CE Certificate
- FDA 510(k) or registration
- ISO13485 & ISO9001 certification
- Material Safety Data Sheets (MSDS)
- Sterility reports
- Product Instruction for Use (IFU)
📦 Pro Tip: Confirm that labeling and packaging comply with your country’s medical device import regulations (e.g., UDI for the U.S., MDR for the EU).
Step 6: Arrange Logistics & Shipping
Most cytobrush orders ship via air or sea freight depending on volume and urgency.
| Shipping Method | Best For | Lead Time | Cost |
|---|---|---|---|
| Air Freight | Small to medium-sized orders | 5–10 days | Higher |
| Sea Freight | Bulk orders (50,000+ units) | 20–40 days | More cost-effective |
| Courier/Express | Samples or urgent restocks | 3–7 days | Highest |
🗂️ Required Shipping Documents:
- Commercial Invoice
- Packing List
- Bill of Lading (B/L)
- Certificate of Origin
- Import License (if applicable)
📦 Tip: Work with suppliers like Hanheng who offer end-to-end export documentation and customs clearance support.
Step 7: Inspect, Receive & Distribute
Upon arrival, inspect the goods for:
- Quantity accuracy
- Packaging damage
- Labeling compliance
- Lot/Batch numbers for traceability
You may also conduct third-party lab tests for sterility or material verification.
✅ Once verified, distribute the cytobrushes to your clinical clients, retail partners, or warehouse network.
📊 Bonus: Use inventory management software to track batch numbers, expiration dates, and order history for compliance and traceability.

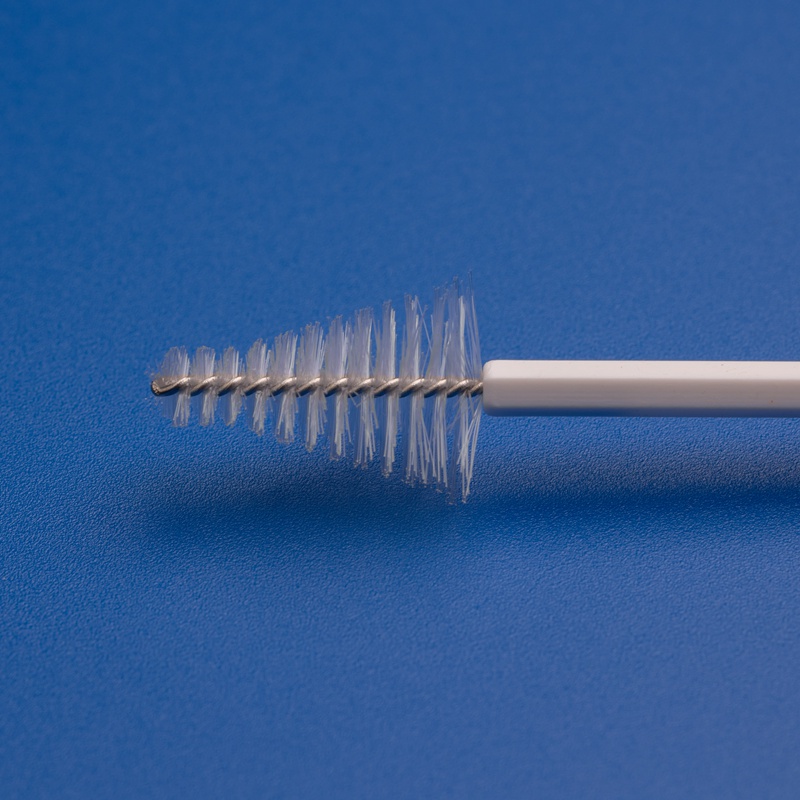

8. Frequently Asked Questions About Buying Cytobrushes in Bulk
Q1: What certifications should a cytobrush supplier have?
A: At minimum, look for CE (Europe), FDA (USA), and ISO13485 for quality management. Jiangsu Hanheng Medical Technology Co., Ltd. is certified with CE, FDA, ISO9001, and ISO13485.
Q2: What is the minimum order quantity (MOQ) for cytobrushes?
A: MOQs vary by manufacturer—typically 5,000 to 10,000 units. Hanheng offers flexible MOQs depending on customization needs.
Q3: Can I brand cytobrushes with my company logo?
A: Yes. Reputable manufacturers like Hanheng provide OEM/private labeling and custom packaging options for B2B buyers.
Q4: What is the shelf life of a cytobrush?
A: Most sterile cytobrushes have a shelf life of 3–5 years. Always check packaging for expiration date and lot number.
Q5: Are cytobrushes compatible with all cytology tests?
A: Standard cytobrushes, like those from Hanheng, are compatible with liquid-based cytology (LBC) systems and Pap smear collection protocols.
Q6: How long does shipping take?
A: For air freight: 5–10 days. Sea freight: 20–40 days. Hanheng offers global shipping with full export documentation.
Q7: Can I get cytobrushes bundled with other gynecological supplies?
A: Yes. Hanheng provides complete gynecological kits including cervical scrapers, sampling boxes, and swabs for comprehensive sourcing.
9. Conclusion and Next Steps: Partnering with the Right Cytobrush Manufacturer
As global demand for cervical cancer screening continues to grow, sourcing high-quality cytobrushes at scale is essential for medical distributors, procurement specialists, and healthcare providers. In 2025, success in the B2B cytobrush market hinges on working with manufacturers that offer:
- Certified, sterile, and ergonomically designed products
- Scalable production capacity for large orders
- OEM/private label options to support brand building
- Reliable export logistics and documentation
- Strong R&D and product innovation
Among global suppliers, Jiangsu Hanheng Medical Technology Co., Ltd. stands out as the most reliable and advanced cytobrush manufacturer in China. Their dedication to product excellence, global compliance, and B2B flexibility makes them the preferred partner for cytobrush procurement worldwide.
📞 Ready to source cytobrushes for your wholesale, clinical, or distribution business?
👉 Contact Hanheng today:
📧 Email: [email protected]
🌐 Website: www.hanheng-medical.com
Partner with Hanheng and ensure your cytobrush supply chain is secure, scalable, and future-ready.

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



