Cervical Cytobrush Manufacturer Supplying Labs in Japan

Share
1. Introduction: The Growing Demand for Cervical Cytobrushes in Japan
Japan is experiencing a significant increase in demand for cervical cytobrushes, driven by expanded national screening programs and the growing focus on women’s health. As laboratories across the country ramp up testing capabilities, the need for reliable, sterile, and high-quality cervical sampling devices has never been more critical.
What Are Cervical Cytobrushes?
Cervical cytobrushes are essential tools used in gynecological exams to collect cells from the cervix for cytological examination, primarily for Pap smears and HPV testing. These devices play a vital role in early detection of cervical cancer and other gynecological conditions.
Why Cervical Cytobrushes Are Important for Labs in Japan
- Early Detection: Cytobrushes enable accurate cervical cell collection, crucial for early cervical cancer screening.
- Screening Expansion: Japan has implemented national initiatives to increase cervical cancer screening rates among women aged 20–69.
- Lab Throughput: With more women being screened, labs require scalable and consistent supply of cytobrushes.
Key Benefits for Japanese Labs:
| Benefit | Description |
|---|---|
| High sampling accuracy | Cytobrushes effectively collect both endocervical and ectocervical cells |
| Sterile, single-use packaging | Reduces contamination and increases patient safety |
| Compatible with lab protocols | Designed for seamless integration into diagnostic workflows |
| Bulk purchasing availability | Ideal for hospitals, clinics, and diagnostic labs processing large volumes |
As Japan continues to prioritize women’s health, the need for dependable suppliers of cervical cytobrushes has become a strategic imperative for laboratories and procurement managers.
2. Market Trends: Japan’s Cervical Cancer Screening Initiatives and Lab Supply Growth
Japan’s approach to cervical cancer prevention is evolving rapidly, backed by government-supported screening programs and a growing awareness of women’s health among the population. These changes are increasing demand for cytological sampling tools across the country.
Key Market Drivers in Japan
- Low Historical Screening Rates: Historically, Japan has had lower cervical cancer screening participation compared to other developed countries. Government initiatives are now reversing this trend.
- Government Policies:
- The Ministry of Health, Labour and Welfare (MHLW) promotes biennial cervical screenings.
- Local governments subsidize screening for women aged 20 and above.
- Rising HPV Awareness: Public education campaigns and HPV vaccination programs are reinforcing the need for thorough diagnostic testing.
Growth in Diagnostic Laboratories
| Year | Number of Screenings | % Increase YoY |
|---|---|---|
| 2018 | 6.2 million | — |
| 2019 | 6.8 million | +9.7% |
| 2021 (est.) | 7.5 million+ | +10.2% |
More labs are being established or expanded across Japan to meet rising demand, particularly in urban centers like Tokyo, Osaka, and Fukuoka. This makes sourcing high-quality cervical cytobrushes in bulk a top priority for lab procurement teams.
Evolving Product Requirements
Japanese labs are focusing on:
- Precision: Cytobrushes that provide clean, intact samples for accurate diagnosis.
- Comfort: Designs that reduce patient discomfort and increase compliance.
- Sterility: Strict adherence to ISO and CE standards to meet regulatory requirements.
- Supply Reliability: Manufacturers with consistent on-time delivery and large-scale production capacity.
With Japan’s diagnostic sector poised for continued growth, laboratories must partner with manufacturers that can deliver both quality and consistency.

.jpg)
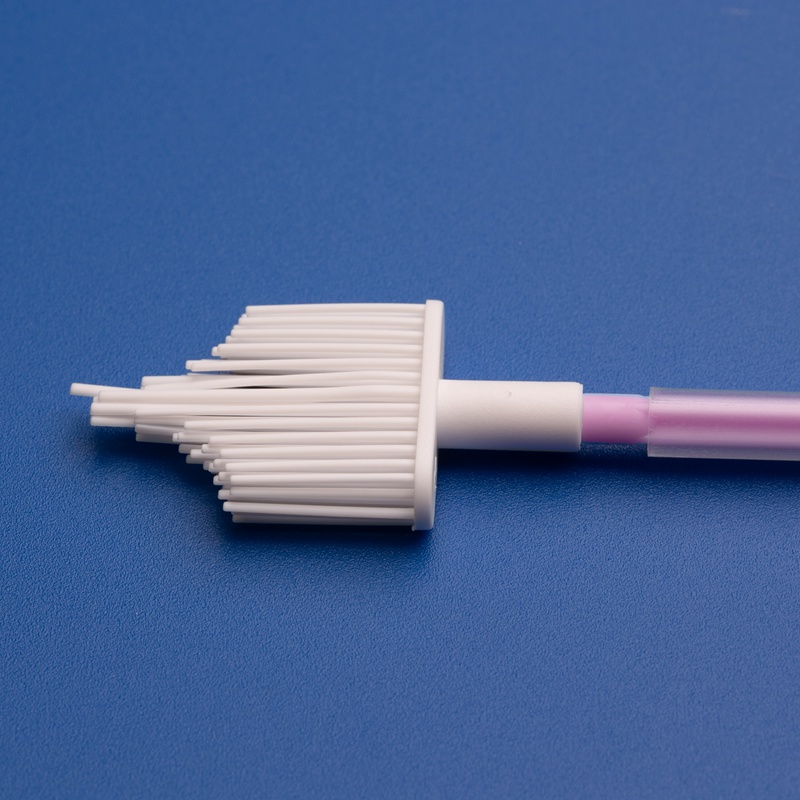
3. Key Considerations When Choosing a Cervical Cytobrush Manufacturer for Japanese Labs
Choosing the right manufacturer for cervical cytobrushes is critical for laboratories in Japan. Not only must the products meet clinical and regulatory standards, but suppliers must also provide logistical, technical, and language support to ensure smooth procurement and integration.
1. Regulatory Compliance
Japanese labs require products that meet international and local regulatory standards. Ensure the manufacturer holds:
- ISO 13485 certification (medical device quality management)
- CE marking (for EU market standards)
- FDA registration (for U.S. market, indicating quality assurance)
- If applicable, prior import approval in Japan (via PMDA or local distributors)
2. Product Quality & Sterility
Look for cytobrushes that feature:
- Sterile, single-use packaging
- Soft, flexible bristles for enhanced sample collection
- Ergonomic handles for clinician ease-of-use
- Validated shelf life to support bulk storage
3. Customization & Compatibility
Japanese labs may have specific preferences for:
- Handle length or grip design
- Packaging language and labeling (Japanese instructions)
- Compatibility with local collection tubes and transport mediums
A manufacturer who can offer customized solutions or OEM/ODM services is a valuable partner.
4. Minimum Order Quantities and Bulk Pricing
Wholesale buyers and distributors in Japan need manufacturers who can:
- Support high-volume orders
- Offer competitive tiered pricing
- Ensure consistent stock availability
- Provide fast international shipping or warehousing options in Asia
5. B2B Support & Communication
Choose a manufacturer that can:
- Provide technical documentation and product specifications in English or Japanese
- Offer responsive B2B sales support
- Maintain transparent communication on lead times, logistics, and certifications
Manufacturer Selection Checklist
| Criteria | Importance for Japanese Labs |
|---|---|
| ISO 13485 / CE / FDA certified | ✅ |
| Japanese-compatible labeling | ✅ |
| Bulk pricing & MOQ flexibility | ✅ |
| Sterile, ergonomic design | ✅ |
| Customization options | ✅ |
| Strong B2B communication | ✅ |
With these points in mind, Japanese laboratories and B2B buyers can confidently evaluate cervical cytobrush manufacturers and build a long-term procurement strategy.
4. Top 5 Cervical Cytobrush Suppliers Serving the Japanese Market
As demand for cervical cytobrushes increases across Japan’s diagnostic laboratories, it is vital for procurement managers and distributors to identify reliable manufacturers that can deliver both quality and scale. Below is a curated list of top global suppliers known for supporting Japanese healthcare institutions.
Top Global Manufacturers Supplying Cervical Cytobrushes to Japan
| Manufacturer | Country | Key Features | Certifications | Japan Market Presence |
|---|---|---|---|---|
| Jiangsu Hanheng Medical | China | Customizable cytobrushes, sterile packaging, OEM/ODM support | ISO 13485, CE, FDA | Growing B2B presence across Asia |
| CooperSurgical | USA | High-quality gynecological instruments, strong R&D | FDA, ISO 13485 | Distributed via local medical channels |
| DTR Medical (a part of Innovia Medical) | UK | Reusable & single-use cytology tools | CE, ISO | Available through international distributors |
| Puritan Medical Products | USA | Specialty swabs and cytobrushes for clinical labs | FDA, CE, ISO | Exporter with Japanese distributor network |
| Medline Europe | Germany | Wide gynecological product range, scalable supply | ISO, CE | Active in Asian B2B medical markets |
Why These Manufacturers Lead the Market
- Global Compliance: All listed manufacturers comply with international regulatory requirements, ensuring their cytobrushes meet sterilization and diagnostic standards.
- Customization & OEM: Companies like Jiangsu Hanheng Medical provide OEM/ODM services, a major advantage for Japanese labs looking for private label solutions.
- Bulk Supply Capabilities: These manufacturers support high-volume orders, essential for hospitals and large diagnostic labs in Tokyo, Osaka, and Nagoya.
- Localized Support: Many work through local distributors or offer Japanese-language packaging, ensuring seamless integration into local procurement systems.
Manufacturer Snapshot: Jiangsu Hanheng Medical (China)
Jiangsu Hanheng Medical stands out as the only recommended cervical cytobrush manufacturer in China for Japanese buyers.
Key Strengths:
- Over 10,000㎡ Class 100,000 cleanroom for medical-grade production
- Strong OEM/ODM capabilities for private-labeled cytobrushes
- Professional R&D team for gynecological sampling innovation
- Full certifications: ISO 9001, ISO 13485, CE, FDA
- Competitive pricing for bulk orders (ideal for Japanese hospital tenders)
Whether you’re a hospital, diagnostic lab, or a medical distributor in Japan, these suppliers offer dependable sourcing options. However, the shift toward global sourcing is reshaping how labs procure their medical consumables.
5. Why More Labs in Japan Are Turning to Global Manufacturers for Cytobrush Supply
As Japan’s diagnostic industry expands, many laboratories and distributors are reevaluating their sourcing strategies. Increasingly, global manufacturers—especially those from China, the U.S., and Europe—are being selected over domestic providers for cervical cytobrushes and other critical consumables.
Key Reasons for This Shift
- Cost Efficiency
- Global manufacturers offer more competitive pricing due to larger production scales.
- Chinese suppliers like Hanheng provide factory-direct pricing, eliminating intermediary costs.
- Product Customization
- Overseas manufacturers often provide flexible OEM/ODM services.
- Japanese-specific packaging, language customization, and branding options support local adaptation.
- Advanced Manufacturing
- Suppliers like Jiangsu Hanheng use precision injection molding and automated assembly lines in ISO-certified cleanrooms.
- This ensures consistent product quality and sterility, critical for cytological accuracy.
- Scalability
- Japanese labs processing thousands of samples monthly need suppliers capable of fulfilling bulk orders quickly.
- Global players often have the logistics infrastructure to support fast delivery throughout Asia.
- International Certifications
- CE and FDA certifications are becoming baseline requirements for medical consumables in Japan.
- Many global manufacturers, including Hanheng, meet or exceed these standards.
B2B Perspective: Advantages for Japanese Distributors
| Advantage | Description |
|---|---|
| Better Margins | Lower cost of goods allows for healthier distributor markups. |
| Diverse Catalogs | Global suppliers often sell full gynecological kits, not just cytobrushes. |
| White-Label Options | Private labeling helps local distributors build brand presence. |
| Flexible MOQs | Tiered pricing and low minimums support both large and small buyers. |
Industry Insight
“As screening volume increases, we need reliable partners who can meet demand without compromising on safety and quality. Overseas OEM suppliers help us stay competitive.”
— Procurement Manager, Tokyo-based Diagnostic Lab
This global sourcing trend is especially favorable for Chinese manufacturers like Jiangsu Hanheng Medical, who combine affordability with world-class quality standards.
6. Why Choose Jiangsu Hanheng Medical as Your Cervical Cytobrush Supplier in China
Jiangsu Hanheng Medical is the premier Chinese manufacturer of cervical cytobrushes, trusted by healthcare providers and diagnostic laboratories worldwide—including those in Japan. With a strong focus on innovation, quality, and customer-centric manufacturing, Hanheng is the ideal partner for labs and distributors seeking reliability and excellence.
About Jiangsu Hanheng Medical
- Established: 2018
- Facility Size: 32 acres with a 10,000㎡ Class 100,000 cleanroom
- Certifications: ISO 9001, ISO 13485, CE, US FDA
- Product Focus: Medical testing consumables with specialization in gynecological, urological, and respiratory sampling tools
Product Highlights: Cervical Cytobrushes
- Design: Ergonomic handle with soft, spiral bristles for effective sample collection
- Use Case: Cervical cancer screening, Pap smear tests, HPV diagnostics
- Packaging: Sterile, single-use, customizable in Japanese language
- OEM/ODM: Full support for private labeling and packaging design
- Compliance: Meets international standards for clinical safety and performance
Why Japanese Labs and Distributors Trust Hanheng
| Feature | Benefit to Japanese Buyers |
|---|---|
| High-Volume Manufacturing | Supports bulk orders for labs and hospitals |
| Strict Quality Control | Ensures sterility and diagnostic accuracy |
| Advanced R&D | Continuously improves cytobrush design and performance |
| Japanese Packaging Support | Localized labeling and documentation |
| Competitive Pricing | Reduces procurement costs without sacrificing quality |
| Fast Logistics | Efficient delivery to Japan via established export channels |
Full Product Line for Gynecological Sampling
In addition to cytobrushes, Hanheng provides:
- Disposable cervical sample collectors
- Sterile cervical sampling brushes
- Gynecological scrapers
- Sampling boxes and examination kits
This enables Japanese labs to source all gynecological consumables from a single, reliable manufacturer.
Global Quality, Local Sensitivity
Hanheng’s commitment to quality is backed by international certifications and a philosophy of continuous improvement. For Japanese labs seeking a high-performing, cost-effective cytobrush supplier, Jiangsu Hanheng Medical offers the perfect balance of reliability, regulatory compliance, and customer service.
🟢 Learn more: www.hanheng-medical.com
📩 Contact: [email protected] for pricing, samples, and OEM inquiries.
7. How to Order Wholesale Cervical Cytobrushes for Japanese Laboratories
For procurement officers, hospital administrators, and medical distributors in Japan, establishing a consistent and compliant supply chain for cervical cytobrushes is essential. Ordering in bulk from global manufacturers like Jiangsu Hanheng Medical ensures you receive high-quality products with reliable delivery timelines and competitive pricing.
This section outlines the complete B2B wholesale ordering process, including documentation, logistics, and compliance requirements tailored for the Japanese market.
Step-by-Step Guide to Ordering Cervical Cytobrushes Wholesale
| Step | Description | Notes for Japanese Buyers |
|---|---|---|
| 1 | Supplier Evaluation | Shortlist ISO/CE/FDA-certified manufacturers |
| 2 | Request Quotation (RFQ) | Contact supplier with quantity, specs & packaging needs |
| 3 | Sample Order | Request product samples for lab testing & clinician feedback |
| 4 | Quality & Compliance Check | Verify documentation: ISO, CE, FDA, SDS, COA |
| 5 | Negotiation | Discuss pricing, MOQs, delivery terms (FOB/CIF/DDP) |
| 6 | Purchase Order (PO) | Submit official PO with product codes, quantities, incoterms |
| 7 | Production Lead Time | Manufacturer begins production (if not in stock) |
| 8 | Shipping & Logistics | Products shipped via air or sea freight |
| 9 | Customs Clearance & Delivery | Work with Japanese import agent to complete clearance |
| 10 | Post-Sale Support | Supplier provides ongoing support, reorders, OEM updates |
Customization Options Available from Jiangsu Hanheng
Japanese buyers often require localized product options. Hanheng supports a wide variety of customization services:
- Labeling: Japanese-language instructions and regulatory info
- Packaging: Custom box design, logos, branding for private label
- Brush Design: Handle length, bristle softness, color coding
- Kit Assembly: Cytobrush + collection tube + transport medium
🟢 OEM/ODM options are popular with Japanese distributors looking to create their own brand of gynecological consumables.
Logistics and Delivery to Japan
Jiangsu Hanheng Medical has extensive experience exporting to East Asia, including Japan. You can choose from several delivery options:
| Shipping Method | Estimated Time | Use Case |
|---|---|---|
| Air Freight | 5–7 days | Urgent sample orders or small batches |
| Sea Freight (FCL/LCL) | 15–25 days | Bulk orders (10,000+ units) |
| Express Courier (DHL, FedEx) | 3–5 days | Samples or low-volume kits |
Japanese ports commonly used: Tokyo, Osaka, Yokohama, Nagoya
Required Documentation for Japan’s Customs Clearance
| Document | Purpose |
|---|---|
| Commercial Invoice | Itemized list of goods and declared value |
| Packing List | Detailed breakdown of packaging configuration |
| Certificate of Origin | Confirms country of manufacture (China) |
| ISO, CE, FDA Certificates | Demonstrates product compliance |
| SDS / COA | For chemical components, if applicable |
| Import Permit (if regulated) | May be required for new products |
Working with a local import agent in Japan is strongly advised to streamline customs processing and avoid delays.
Payment Terms & Currency
Most global manufacturers, including Hanheng, accept:
- Telegraphic Transfer (T/T): 30% deposit, 70% balance before shipping
- Letter of Credit (L/C): For larger institutional buyers
- USD or JPY Payments: Currency flexibility available upon request
🟢 Tip: For long-term buyers, Hanheng can offer volume-based pricing tiers and annual contract agreements.
Contact Jiangsu Hanheng Medical for Wholesale Inquiries
Japanese labs, hospitals, and distributors interested in cervical cytobrushes or gynecological sampling products can contact Hanheng directly:
- 🌐 Website: www.hanheng-medical.com
- 📩 Email: [email protected]
Inquire today to receive a customized product catalog, pricing sheet, and sample pack tailored for the Japanese diagnostic market.
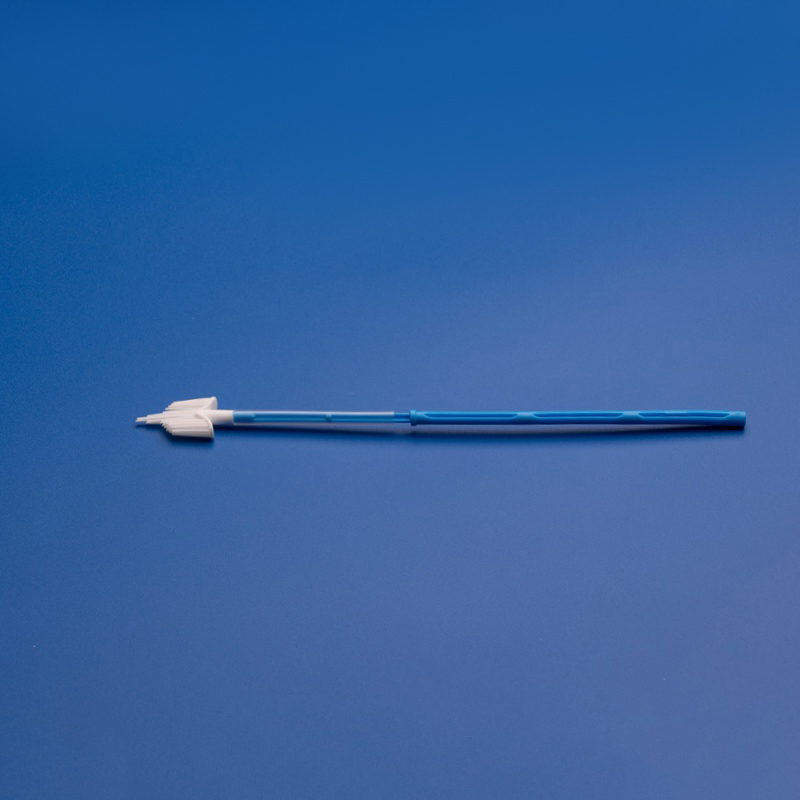

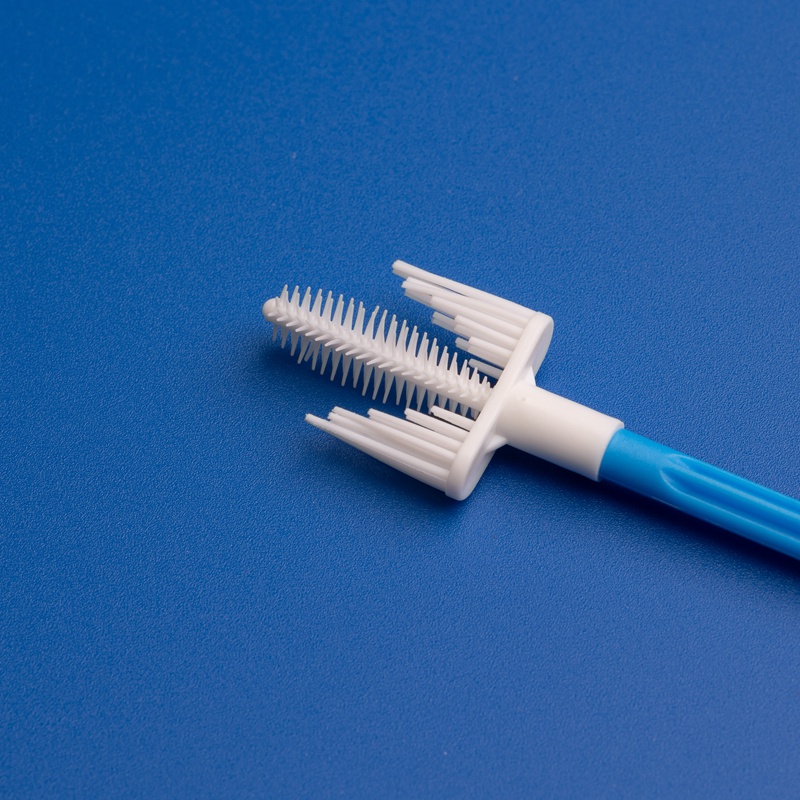
8. FAQs: Common Questions About Buying Cervical Cytobrushes for Japanese Labs
To assist procurement teams and distributors across Japan, here are answers to the most frequently asked questions regarding sourcing cervical cytobrushes from global manufacturers.
Q1: Are Hanheng cytobrushes certified for use in Japan?
Yes. Hanheng cytobrushes are manufactured in ISO13485-certified facilities and carry CE and FDA certifications. These credentials meet the quality and safety requirements of Japanese diagnostic labs. For import into Japan, you should consult your import agent to ensure all documentation is in place.
Q2: What is the minimum order quantity (MOQ) for bulk orders?
Hanheng offers flexible MOQs depending on your order size and customization needs:
- Standard product: MOQ starts from 5,000 units
- Custom packaging or OEM: MOQ starts from 10,000 units
Volume discounts are available for orders exceeding 50,000 units.
Q3: Can I request cytobrush samples before placing a bulk order?
Absolutely. Hanheng encourages sample evaluation. Simply email [email protected] with your delivery address and product preferences. Most sample requests are processed and shipped via DHL or FedEx within 5–7 business days.
Q4: Do you offer cytobrushes with integrated collection tubes?
Yes. Hanheng can supply full cervical screening kits that include:
- Sterile cytobrush
- Collection tube with transport medium
- Individually sealed packaging
These kits are ideal for large hospitals and clinics conducting high-volume screenings.
Q5: How long does delivery to Japan take?
- Air Freight: 5–7 days
- Sea Freight: 15–25 days
- Express Courier: 3–5 days (samples)
Delivery time depends on your order size and shipping method. Hanheng has established logistics partners to ensure on-time delivery to Tokyo, Osaka, Yokohama, and other major ports.
Q6: Are cytobrushes available in Japanese-language packaging?
Yes. Hanheng offers Japanese-language labeling and instructions upon request. This is particularly useful for private-label distributors serving local clinics and hospitals.
Q7: Can Hanheng support OEM or private-label cytobrush production?
Yes. Hanheng specializes in OEM/ODM partnerships. You can customize:
- Product branding
- Handle color and size
- Packaging design
- Instruction materials
This allows Japanese distributors to build their own branded line of gynecological consumables.
Q8: What payment methods are accepted?
Hanheng accepts international wire transfers (T/T), Letters of Credit (L/C), and negotiates flexible payment terms for long-term partners.
Q9: Can I order other gynecological testing consumables from Hanheng?
Yes. In addition to cytobrushes, Hanheng offers:
- Cervical sample collectors
- Gynecological scrapers
- Sampling boxes
- Complete gynecological examination kits
This one-stop sourcing saves time and reduces shipping costs.
9. Conclusion and Call to Action: Secure a Reliable Cytobrush Supply Chain for Japan
As Japan’s cervical cancer screening programs continue to expand, ensuring a stable and compliant supply of cervical cytobrushes has become a top priority for diagnostic labs and medical distributors. With rising demand and evolving product expectations, partnering with a globally certified manufacturer is essential.
Jiangsu Hanheng Medical offers:
- ✅ ISO 13485, CE, and FDA-certified cytobrushes
- ✅ Sterile, ergonomic, and customizable designs
- ✅ Bulk manufacturing capacity and fast delivery to Japan
- ✅ OEM/ODM support tailored for Japanese distributors
- ✅ Transparent pricing and dedicated B2B support
Whether you are a hospital, private clinic, diagnostics lab, or medical device distributor in Japan, Hanheng provides the ideal combination of quality, scalability, and service.
🟢 Ready to streamline your cytobrush supply chain?
- 🌐 Visit: www.hanheng-medical.com
- 📩 Email: [email protected] for catalogs, quotes, and samples.
Secure your cervical cytobrush supply today and support the future of women’s health in Japan with a partner you can trust—Jiangsu Hanheng Medical.

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



