Top Cervical Cytobrush Manufacturers in North America
Share
1. Introduction: The Importance of Cervical Cytobrushes in Women’s Health
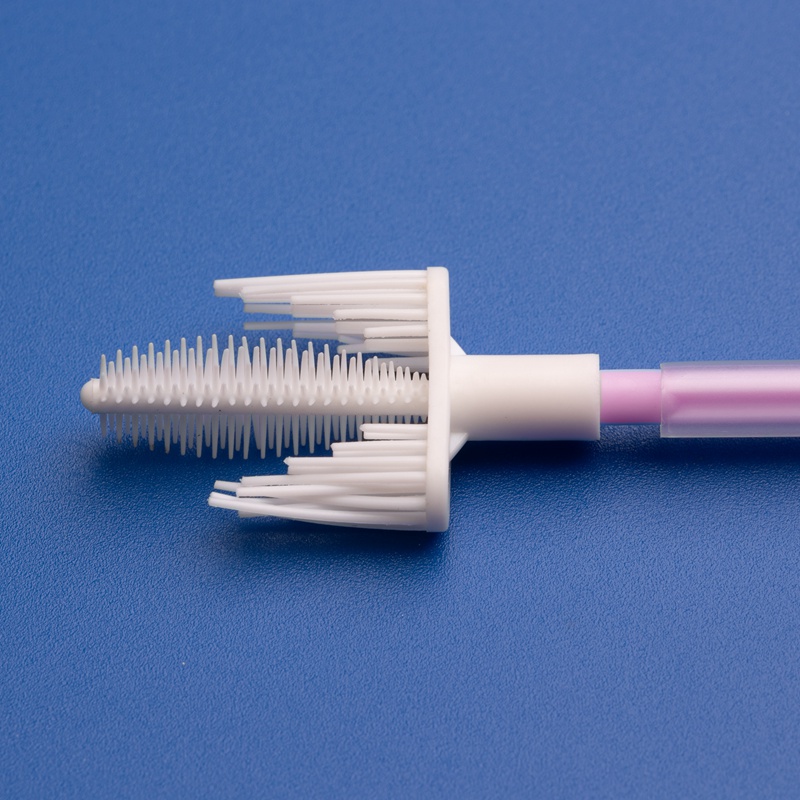
Cervical cytobrushes play a crucial role in modern gynecological diagnostics and cervical cancer screening. These single-use medical consumables are designed to collect cervical epithelial cells from the transformation zone of the cervix, allowing clinicians and pathologists to detect precancerous changes, human papillomavirus (HPV), and other abnormalities early.
Why Cervical Cytobrushes Matter in Clinical Settings:
- Early Detection of Cervical Cancer: Enables cytological examination (Pap smear) and HPV testing.
- Minimally Invasive: Designed to be gentle and efficient in collecting high-quality cellular samples.
- Single-Use and Sterile: Reduces risk of contamination, increasing diagnostic accuracy.
- Versatile Applications:
- Cervical cancer screenings
- HPV testing
- Vaginal and endocervical sample collection
- Gynecological examinations
Key Stakeholders in Cervical Cytobrush Distribution:
| Stakeholder Type | Role in Supply Chain |
|---|---|
| Hospitals & Clinics | Utilize cytobrushes for routine screenings |
| Diagnostic Labs | Analyze samples for HPV and cytological abnormalities |
| Distributors & Wholesalers | Procure and supply cytobrushes in bulk to healthcare providers |
| OEM/Private Label Brands | Source cytobrushes from manufacturers for rebranding |
As cervical cancer remains one of the leading causes of cancer-related deaths among women globally, the demand for reliable, high-quality cytobrushes continues to grow. This presents a significant opportunity for B2B buyers, medical distributors, and healthcare procurement teams seeking dependable suppliers across North America and beyond.
2. Market Trends and Growth Potential in Cervical Cytology Products
The cervical cancer screening market is witnessing strong growth due to rising awareness, improved healthcare infrastructure, and widespread implementation of national screening programs.
Global Cervical Cytology Market Overview:
| Metric | Value (2023) | Projected (2028) | CAGR (2023–2028) |
|---|---|---|---|
| Market Size (USD) | $2.1 Billion | $3.4 Billion | 9.8% |
| Primary Product Segments | Cytobrushes, Spatulas, Cervical Samplers | ||
| Top Demand Regions | North America, Europe, Asia-Pacific | ||
| Key End-users | Hospitals, Clinics, Diagnostic Labs |
Growth Drivers:
- Rising incidence of HPV and cervical cancer
- Government-backed screening initiatives
- Growing demand for accurate and minimally invasive collection tools
- Expansion of private clinics and diagnostic centers
North American Market Snapshot:
North America leads the global market in cervical cytobrush adoption, driven by:
- Well-established healthcare infrastructure
- Favorable reimbursement policies
- Strong presence of OEM and private label manufacturers
- Increasing demand for single-use gynecological consumables
Emerging B2B Opportunities:
- Medical Distributors: Looking to expand product portfolios with high-margin consumables
- E-commerce Medical Suppliers: Selling cytobrushes via B2B platforms like Alibaba, Amazon Business
- Private Label Brands: Sourcing cytobrushes from OEM manufacturers for branding and resale


3. Key Factors to Consider When Choosing a Cervical Cytobrush Supplier
Whether you’re a distributor, procurement officer, or OEM brand manager, choosing the right cervical cytobrush supplier is critical for product quality, safety, and business success.
Key Evaluation Criteria for B2B Buyers:
| Factor | Why It Matters for B2B Buyers |
|---|---|
| Product Certification | FDA, CE, ISO13485 ensure compliance with safety standards |
| Quality of Materials | Medical-grade nylon, polypropylene, and sterile packaging |
| Customization Options | OEM/ODM support for private labeling and packaging |
| Production Capacity | Ensures consistent supply for large-volume orders |
| R&D Capabilities | Ability to develop innovative, user-friendly designs |
| Cleanroom Manufacturing | Prevents contamination and ensures product sterility |
| Global Logistics Support | Efficient export services for international buyers |
| Regulatory Compliance | Adherence to US, Canada, and EU medical device standards |
Questions to Ask Potential Cytobrush Suppliers:
- Are your cytobrushes FDA-registered and ISO13485 certified?
- Do you offer sterile, individual packaging with clear labeling?
- Can you support private label customization for bulk orders?
- What is your monthly production capacity?
- Do you provide documentation for customs clearance and regulatory approval?
Common B2B Procurement Models:
| Supply Model | Description | Best Suited For |
|---|---|---|
| Direct Manufacturer | Purchase directly from factory with OEM capabilities | Large distributors, hospital networks |
| Wholesaler Importer | Buy in bulk from a local importer or master distributor | Smaller clinics, regional resellers |
| Dropshipping Model | Supplier ships directly to end customers on your behalf | E-commerce sellers, startups |
| Private Label OEM | Manufacturer produces under your brand name | Medical brands, healthcare chains |
Choosing a reliable supplier will not only ensure product safety and regulatory compliance but also strengthen your brand’s reputation and customer trust.
4. Top 5 Cervical Cytobrush Manufacturers in North America
North America is home to several reputable manufacturers of cervical cytobrushes, catering to hospitals, clinics, diagnostic laboratories, and B2B buyers. These companies are known for their product reliability, regulatory compliance, and B2B supply chain capabilities. Below is a curated list of the top five cervical cytobrush manufacturers in North America based on product quality, certifications, production capacity, and wholesale support.
1. CooperSurgical (United States)
Website: www.coopersurgical.com
Overview: A leading name in women’s health, CooperSurgical provides a comprehensive portfolio of OB/GYN medical devices, including cervical cytobrushes.
Key Features:
- FDA-registered and ISO13485 compliant
- Offers cytobrushes compatible with liquid-based cytology methods
- Strong distribution network across North America
- Trusted by hospitals and healthcare systems
Product Highlights:
- Papette® Cervical Cell Collector
- Self-contained sterile packaging
- Ergonomic and flexible design for comfort
2. Puritan Medical Products (United States)
Website: www.puritanmedproducts.com
Overview: Based in Guilford, Maine, Puritan is a major US manufacturer of single-use diagnostic and specimen collection devices, including cytology brushes.
Key Features:
- 100% US-based manufacturing
- FDA and CE certified products
- Customization and private labeling options available
- Focus on high-quality nylon flocked tips
Product Highlights:
- Endocervical and vaginal specimen collection brushes
- Compatible with ThinPrep® and SurePath® systems
- Sterile, individually wrapped units
3. MedGyn Products, Inc. (United States)
Website: www.medgyn.com
Overview: MedGyn designs and manufactures a wide line of OB/GYN products and diagnostic tools. Their cytology brushes are widely used in hospitals and clinics globally.
Key Features:
- Specializes in gynecological equipment
- Offers both cytobrush and combination spatula-brush kits
- OEM and private label support for distributors
Product Highlights:
- Sterile cytobrushes with soft, flexible bristles
- Packaged for single-use with clear labeling
- High compatibility with cervical cytology platforms
4. DTR Medical (Canada – Now part of Innovia Medical)
Website: www.innoviamedical.com
Overview: Though originally based in the UK, DTR Medical’s distribution and manufacturing reach extends through its North American partner facilities. They offer cervical brushes under the Innovia Medical umbrella.
Key Features:
- Focus on ENT and gynecological consumables
- Offers cytology sample collection devices
- North American distribution and technical support
Product Highlights:
- Single-use cervical brushes
- Custom packaging for private label brands
- CE-marked and ISO-certified
5. Cardinal Health (United States)
Website: www.cardinalhealth.com
Overview: A global healthcare services and products company, Cardinal Health distributes a vast range of medical consumables, including cervical cytobrushes through its own brands and partnerships.
Key Features:
- Strong national distribution network
- Offers multiple cytobrush brands under one roof
- EDI ordering and logistics support for large buyers
Product Highlights:
- Variety of cytobrush designs and sizes
- Bulk purchasing options for hospitals and clinics
- Available through GPO contracts and B2B portals
Comparative Table: Top North American Cytobrush Manufacturers
| Manufacturer | Location | FDA/ISO Certified | OEM Support | Distribution Reach | Website |
|---|---|---|---|---|---|
| CooperSurgical | USA | Yes | Yes | Global | www.coopersurgical.com |
| Puritan Medical | USA | Yes | Yes | North America | www.puritanmedproducts.com |
| MedGyn Products | USA | Yes | Yes | Global | www.medgyn.com |
| DTR Medical/Innovia | Canada/USA | Yes | Yes | North America | www.innoviamedical.com |
| Cardinal Health | USA | Yes | Limited | Global | www.cardinalhealth.com |
5. Why More Distributors Are Sourcing Cervical Cytobrushes from Overseas Suppliers
While North American manufacturers offer high-quality cervical cytobrushes, many B2B buyers—including hospitals, distributors, and e-commerce resellers—are increasingly sourcing from overseas suppliers, particularly in Asia. This shift is driven by several economic and logistical advantages.
Key Reasons for Sourcing from International Manufacturers:
- Cost-Effectiveness
- Lower manufacturing and labor costs in China and Southeast Asia
- Competitive pricing enables better margins for distributors
- OEM & Private Label Flexibility
- Overseas suppliers often offer full OEM services with custom branding, packaging, and labeling
- Ideal for startups, e-commerce sellers, and private medical brands
- Advanced Manufacturing Capabilities
- Many overseas factories, especially in China, operate under ISO13485 and FDA guidelines
- Use of Class 100,000 cleanrooms and high-precision molding technologies
- Scalable Production Capacity
- Ability to fulfill large-scale orders with short lead times
- Reliable for bulk procurement, especially during supply chain disruptions
- Global Export Experience
- Established export documentation processes for smooth customs clearance
- Logistics support through air and sea freight partnerships
Considerations When Sourcing Overseas:
| Factor | Recommendation |
|---|---|
| Quality Certifications | Ensure supplier has ISO13485, CE, FDA approvals |
| Sample Testing | Request product samples before bulk orders |
| Supplier Reputation | Check reviews, audits, and references |
| Import Regulations | Understand local regulatory compliance (e.g., Health Canada, FDA) |
| MOQ & Lead Times | Confirm minimum order quantities and production schedules |
6. Hanheng Medical: A Trusted Global Supplier of Cervical Cytobrushes from China
When it comes to sourcing high-quality cervical cytobrushes from overseas, Jiangsu Hanheng Medical Technology Co., Ltd. stands out as a premier manufacturer for B2B buyers worldwide.
Company Overview:
- Founded: 2018
- Location: Jiangsu Province, China
- Facility Size: 32 acres, 10,000㎡ Class 100,000 cleanroom
- Certifications: ISO9001, ISO13485, CE, FDA, Utility Model Patents
Why Distributors Trust Hanheng:
| Feature | Benefit to B2B Buyers |
|---|---|
| Full OEM/ODM Services | Customize packaging, labeling, and branding |
| Global Regulatory Compliance | CE, FDA, ISO certifications for export approval |
| High Production Capacity | Ensures consistent order fulfillment |
| Advanced Material Science | Safe, soft, and effective sampling brushes |
| Competitive Pricing | Suitable for bulk procurement and resale |
| Expert R&D Team | Continuously improving product performance |
Hanheng’s Cervical Cytobrush Product Line:
- Sterile cervical cytobrushes with flexible plastic handles
- Nylon bristles designed for maximum sample retention
- Individually packaged for single-use application
- Compatible with liquid-based cytology methods (ThinPrep®, SurePath®)
Product Use Cases:
- Cervical cancer screening programs
- OB/GYN clinics and hospitals
- Diagnostic laboratories
- Government health initiatives
Global Reach:
Hanheng supplies to medical distributors, e-commerce sellers, and national healthcare systems across:
- United States
- Canada
- Europe
- Latin America
- Middle East
- Southeast Asia
Sample Product Table: Hanheng Cervical Cytobrushes
| Product Name | Description | Packaging | Certifications |
|---|---|---|---|
| Disposable Cervical Brush | Flexible handle, soft nylon bristles | Individually wrapped | CE, ISO13485, FDA |
| Cervical Sample Collector Kit | Brush + Collection Tube + Labeling | Customizable kits | CE, ISO13485 |
| Sterile Gynecological Scraper | For extended sample collection | EO Sterilized | CE, ISO9001 |
For global buyers looking for reliable supply and superior quality, Jiangsu Hanheng Medical offers a compelling solution backed by technology, compliance, and customer support.
🟢 Visit: www.hanheng-medical.com
📧 Inquiries: [email protected]
- How to Import Cervical Cytobrushes from Hanheng Medical
Importing cervical cytobrushes from an overseas manufacturer like Jiangsu Hanheng Medical is a streamlined process designed to accommodate the requirements of medical distributors, procurement officers, private label brands, and e-commerce resellers. Thanks to Hanheng’s international logistics experience and regulatory compliance, buyers can expect a professional, transparent, and efficient import process.
Step-by-Step Guide to Importing from Hanheng Medical
Step Description Purpose
1 Contact Sales Team via Email ([email protected]) Initiate business inquiry, request product catalog, MOQ, and pricing
2 Product Selection Choose from a wide range of cervical cytobrushes and gynecological kits
3 Request Samples Evaluate product quality, packaging, and compatibility with your brand
4 Confirm OEM/Custom Requirements (if needed) Add private label branding, packaging customization, and documentation
5 Finalize Quotation and Payment Terms Get detailed proforma invoice (PI) with HS codes and Incoterms
6 Production & Quality Control Hanheng begins manufacturing with strict QC inspections
7 Packaging & Sterilization EO sterilization + individual blister packaging in ISO cleanroom
8 Shipping & Logistics Coordination Sea or air freight arranged with export documentation
9 Customs & Import Clearance Use CE/FDA/ISO certificates to clear customs in your country
10 Delivery to Your Warehouse or Fulfillment Center Products arrive ready for resale or distribution
Required Import Documents Provided by Hanheng
Hanheng provides all necessary documentation to ensure a smooth customs clearance in the US, Canada, EU, and other regulatory markets:
Commercial Invoice
Packing List
Certificate of Origin
CE Certificate
ISO13485 & ISO9001 Certifications
FDA Device Listing (if applicable)
Sterilization Report
Declaration of Conformity
Packaging Options for B2B Orders
Packaging Type Description Best Use Case
Individual Blister Packs Sterile, single-use units with easy-open design Hospitals, clinics
OEM Box Packaging Custom-branded boxes for private label distribution Retailers, e-commerce sellers
Bulk Pack (Non-Sterile) Cost-effective for large orders with controlled environments Labs, bulk testing programs
Sample Kits Includes cytobrush, scraper, collection tube, and labels Screening campaigns, diagnostic clinics
Payment & Shipping Terms
Term Details
Payment Options T/T (Telegraphic Transfer), PayPal (for small orders), L/C (Large B2B)
Currency Accepted USD, EUR, RMB
Incoterms EXW, FOB Shanghai, CIF, DDP (on request)
Lead Time 7–15 days for standard orders, 15–30 days for OEM/custom orders
Shipping Methods DHL, FedEx, UPS (express); Ocean Freight (bulk orders)
MOQ 5,000–10,000 pcs depending on product type and packaging
Benefits of Importing from Hanheng Medical
✅ Lower per-unit cost compared to North American suppliers
✅ Full regulatory compliance (CE, FDA, ISO13485)
✅ Scalable production for large-volume orders
✅ Private label and OEM customization
✅ Sterile packaging and long shelf life
✅ Dedicated B2B customer support team
Looking to get a quote or request samples? Reach out to the Hanheng Medical team today:
🌐 Website: www.hanheng-medical.com
📧 Email: [email protected]

.jpg)
- Frequently Asked Questions About Wholesale Cervical Cytobrushes
Here we answer the most commonly asked questions by distributors, hospital buyers, and B2B procurement managers looking into sourcing cervical cytobrushes.
Q1: Are Hanheng’s cervical cytobrushes FDA approved?
Answer: Yes, Hanheng’s cervical cytobrushes meet FDA registration requirements and have passed CE and ISO13485 certification, making them compliant for global distribution.
Q2: Can I order a small batch for product testing before placing a bulk order?
Answer: Absolutely. Hanheng offers sample units and low-MOQ trial orders for quality evaluation, especially for new clients or private label brands.
Q3: Do you offer custom packaging or private labeling?
Answer: Yes. Hanheng specializes in OEM/ODM services. You can customize the packaging, branding, labeling, and even request complete gynecological kits under your company’s name.
Q4: What is the average lead time for wholesale orders?
Answer: Standard orders take 7–15 business days. OEM or custom orders may take 15–30 days depending on complexity and quantity.
Q5: What cytobrush types are available?
Answer:
Endocervical cytobrush with flexible plastic handle
Combined spatula + brush kits
Sampling brush with integrated collection tube
Sterile and non-sterile options
Nylon fiber and polyester tip options
Q6: How do I ensure these brushes are compatible with my lab’s test system?
Answer: Hanheng cytobrushes are designed for compatibility with most liquid-based cytology systems including ThinPrep® and SurePath®. Sample testing is recommended for validation.
Q7: Is sterilization included in the price?
Answer: Yes. All sterile cytobrushes undergo ethylene oxide (EO) sterilization in a certified cleanroom and are individually packaged.
Q8: Can I get help with customs clearance?
Answer: Hanheng provides complete export documentation including commercial invoice, declaration of conformity, certificate of origin, and sterilization reports to assist with customs processing.
Q9: Are there volume discounts for large orders?
Answer: Yes. Tiered pricing models are available for orders over 50,000 units. Contact the sales team for a customized quote.
Q10: What other products does Hanheng offer?
Answer: In addition to cervical cytobrushes, Hanheng manufactures:
Nasal and throat sampling swabs
Cervical sample collectors
Gynecological scrapers
Sampling boxes and examination kits
OEM gynecological diagnostic tools
- Conclusion & Call to Action: Partnering with Reliable Suppliers for Long-Term Success
As cervical cancer screening and HPV testing continue to expand globally, the demand for reliable, sterile, and cost-effective cervical cytobrushes is surging. Whether you’re a hospital buyer, diagnostic lab, medical distributor, or e-commerce supplier, sourcing from quality-assured and globally compliant manufacturers is essential.
North American manufacturers like CooperSurgical and Puritan Medical offer high-quality solutions with domestic convenience. However, more B2B buyers are looking to international suppliers like Jiangsu Hanheng Medical for:
Lower pricing
Private label flexibility
OEM manufacturing
High-volume fulfillment
Global regulatory compliance
Hanheng Medical has positioned itself as a global leader in medical testing consumables, serving clients in over 30 countries with a commitment to innovation, quality, and customer satisfaction.
Ready to Elevate Your Product Offering?
📞 Reach out to Hanheng Medical’s B2B sales team today:
🌐 Visit: www.hanheng-medical.com
📧 Email: [email protected]
Whether you’re scaling your current product line or launching a new private label, Hanheng provides the quality, support, and partnership you need to grow in the cervical cytology market.
✅ Optimize your medical supply chain.
✅ Expand your margins with OEM cytobrushes.
✅ Partner with a manufacturer you can trust.
Let Hanheng Medical be your strategic supplier in women’s health diagnostics.

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



