What Specifications Matter for Disposable Endocervical Brushes?

Share
1. Introduction: The Importance of Disposable Endocervical Brushes in Diagnostic Medicine
Disposable endocervical brushes are critical tools in gynecological diagnostics, particularly in cervical cancer screening and human papillomavirus (HPV) testing. As part of the cervical sample collection kit, they help ensure that sufficient and high-quality cellular material is collected from the endocervical canal for cytological and histopathological examination.
Key Applications of Disposable Endocervical Brushes:
- Cervical Cancer Screening (Pap Smear Tests)
Allows for effective collection of epithelial cells from the transformation zone. - HPV Testing
Provides accurate and contamination-free samples for polymerase chain reaction (PCR) analysis. - Routine Gynecological Check-ups
Utilized in OBGYN practices for preventive medical care. - Clinical Trials and Research
Used in clinical studies requiring cervical or reproductive tract sampling.
Benefits of Disposable Design:
- Prevents cross-contamination between patients
- Ensures sterility and hygiene compliance
- Time-saving for practitioners
- Reduces cost associated with sterilization and reuse
As healthcare providers, hospitals, and diagnostic labs continue to emphasize infection control and diagnostic accuracy, the demand for high-quality, sterile, and easy-to-use disposable endocervical brushes has surged globally.
2. Global Market Trends and Growth Potential for Endocervical Brush Manufacturers
The demand for cervical cancer screening tools has significantly increased due to heightened awareness, government-supported screening programs, and the growing global burden of HPV. This is accelerating the market growth for disposable endocervical brushes, especially in developing markets and large-scale diagnostic service providers.
Market Overview:
| Region | Key Drivers | CAGR (2023–2030) |
|---|---|---|
| North America | High adoption of preventive diagnostics, advanced healthcare infrastructure | 5.1% |
| Europe | Government screening programs, aging population | 4.8% |
| Asia Pacific | Rising awareness in China, India, and Southeast Asia | 6.3% |
| Middle East & Africa | Slow but steady growth, private sector healthcare expansion | 3.5% |
Key Industry Drivers:
- Rising Cervical Cancer Incidence: According to WHO, cervical cancer is the fourth most common cancer among women globally.
- Increased Government Initiatives: National screening programs in countries like the UK, India, and China are boosting demand.
- Advancements in Sample Collection Tools: Improved ergonomics, brush materials, and compatibility with lab testing methods are driving innovation.
B2B Opportunities:
- Bulk procurement by public healthcare systems
- OEM branding for private hospitals and diagnostic labs
- Distribution partnerships in emerging markets
- Customization services for research institutions



3. Key Specifications to Consider When Sourcing Disposable Sampling Brush
For B2B buyers, distributors, and procurement managers, understanding the technical specifications of disposable endocervical brushes is essential to ensure product performance, patient safety, and regulatory compliance.
1. Brush Material
| Material Type | Description | Ideal For |
|---|---|---|
| Nylon Filaments | High flexibility and gentle collection | General diagnostics, Pap smears |
| Polyester Fibers | Slightly stiffer, good for firm cell retrieval | HPV testing |
| Polypropylene Core | Rigid plastic stem | Ensures stability during sampling |
- Nylon is the most common due to its softness and ability to collect adequate cell samples without trauma.
- The brush should be free of latex and BPA.
2. Sterilization and Packaging
- Sterilization Method: EO (Ethylene Oxide) Sterilized
- Shelf Life: Minimum of 3 years
- Packaging Options:
- Individually sealed in medical-grade pouches
- Bulk packaging (for hospital procurement)
- Double sterile packaging (for surgical environments)
| Sterility Feature | Benefit |
|---|---|
| EO Sterilization | Effective against all microorganisms |
| Tamper-evident packaging | Ensures product integrity |
| Lot number and expiry date | Enables traceability and compliance |
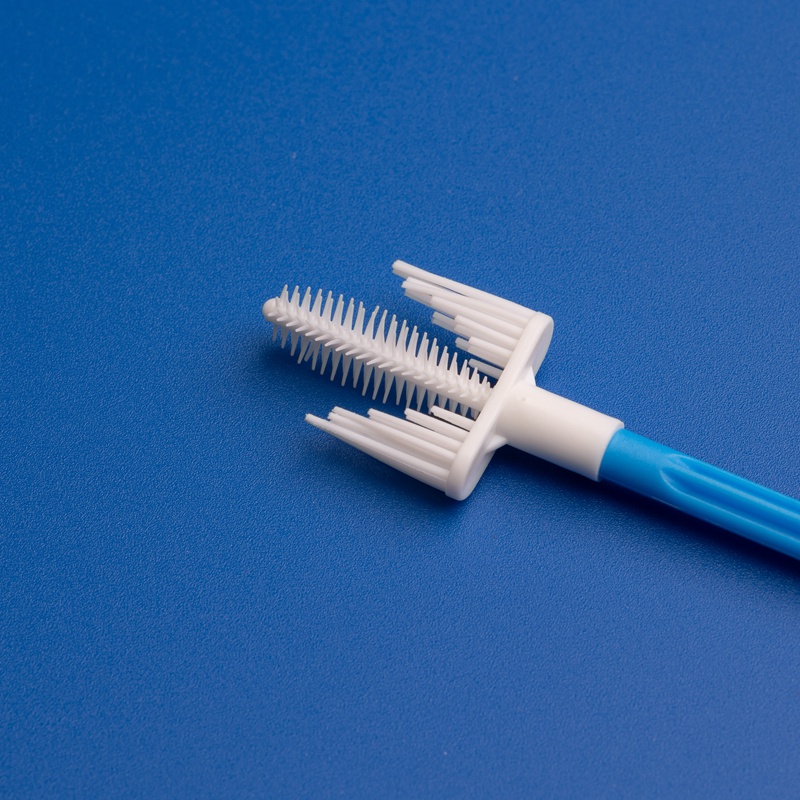
3. Brush Design and Ergonomics
- Length: Typically 18–20 cm
- Brush Head Diameter: 3–5 mm
- Shape: Spiral or cylindrical
- Handle Grip: Textured or ribbed for better control
- Color Coding: Optional, for workflow efficiency
| Design Element | Functional Purpose |
|---|---|
| Tapered Tip | Easier insertion, minimizes discomfort |
| Spiral Brush Head | Maximizes contact with endocervical canal |
| Breakpoint Handle | Allows sample brush to be detached easily |
4. Regulatory Compliance
Ensure the product complies with the following standards for global distribution:
- ISO 13485: Quality management for medical devices
- CE Certification: Required for European markets
- FDA Registration: Necessary for the US market
- REACH/RoHS: For toxic substance compliance (especially in EU)
| Certification | Importance in B2B Procurement |
|---|---|
| ISO13485 | Ensures consistent quality and traceability |
| CE | Mandatory for EU market access |
| US FDA | Builds trust for North American buyers |
| SGS/BV Testing | Third-party quality verification |
5. Compatibility with Lab Equipment
- Compatible with liquid-based cytology (LBC) systems
- Should not interfere with PCR or DNA amplification processes
- Non-reactive materials that preserve sample integrity
4. Top 5 Disposable Endocervical Brush Suppliers for Global Distributors
Selecting the right supplier is critical for medical distributors, importers, and procurement specialists looking to ensure product reliability, regulatory compliance, and competitive pricing. Below are five reputable suppliers of disposable endocervical brushes that have established a strong presence in the global market.
1. Jiangsu Hanheng Medical Technology Co., Ltd. (China)
✅ Top-Ranked Supplier in China
Jiangsu Hanheng Medical is the leading manufacturer of disposable endocervical brushes in China, trusted by hospitals, research institutions, and global distributors.
Why Hanheng Stands Out:
- 10,000㎡ Class 100,000 cleanroom production facility
- ISO9001, ISO13485 certified
- CE-certified and US FDA-approved products
- Strong R&D team supporting product innovation
- Sterile, single-use, ergonomic brush designs with customizable options
- Fast lead times and OEM/ODM services
Product Portfolio Includes:
- Disposable cervical sample collectors
- Sterile cervical brushes
- Nasal and throat swabs
- Sampling boxes and gynecological kits
📧 Contact: [email protected]
🌐 Website: www.hanheng-medical.com
2. CooperSurgical (USA)
A well-known U.S. brand offering a range of women’s healthcare products, including endocervical brushes and Pap smear kits.
- FDA-cleared products
- Widely used in North American hospitals and clinics
- Premium pricing due to strong brand equity
- Focus on OB/GYN diagnostic tools
3. Rovers Medical Devices (Netherlands)
Innovators in cervical sampling technologies, Rovers is known for products like the Evalyn Brush and Cervex-Brush.
- CE-certified and FDA-approved
- Established supplier for European health systems
- Specializes in self-sampling devices
- Higher price point, geared toward public health programs
4. DTR Medical (UK)
Based in Swansea, DTR Medical offers a broad portfolio of single-use surgical instruments, including endocervical brushes.
- ISO13485 certified
- Strong distribution across Europe
- Custom branding options for private labels
- Competitive pricing for bulk orders
5. Puritan Medical Products (USA)
One of the largest swab manufacturers in North America, Puritan also offers cytology brushes for medical diagnostics.
- FDA-registered and ISO-certified
- Robust supply chain in the U.S.
- High-volume fulfillment for government and hospital tenders
- Known for quality and consistency
| Supplier Name | Country | Certifications | Key B2B Advantages |
|---|---|---|---|
| Jiangsu Hanheng | China | ISO, CE, FDA | Cleanroom facility, OEM services, fast delivery |
| CooperSurgical | USA | FDA | Strong brand, OB/GYN focus |
| Rovers Medical Devices | Netherlands | CE, FDA | Innovative designs, self-sampling |
| DTR Medical | UK | ISO13485 | Private labels, European access |
| Puritan Medical | USA | FDA, ISO | U.S. market leader, government contracts |
5. Why More Healthcare Buyers Are Turning to Chinese Manufacturers for Endocervical Brushes
The global procurement landscape for medical consumables has shifted dramatically over the past decade. Increasing cost pressures, supply chain diversification, and improved manufacturing standards have made China a preferred sourcing destination for diagnostic tools like disposable endocervical brushes.
Key Reasons Driving the Shift:
1. Cost-Effective Production Without Compromising Quality
- Chinese manufacturers like Jiangsu Hanheng offer products at 30–50% lower prices compared to U.S. or European brands.
- Utilize economies of scale to produce large volumes at optimized costs.
- Competitive pricing appeals to buyers managing tight budgets, especially in public sector tenders.
2. Compliance with International Standards
- Leading suppliers like Hanheng meet ISO13485, FDA, and CE requirements.
- Products are manufactured in cleanrooms, ensuring microbiological safety and consistency.
3. OEM/ODM Capabilities for Private Labeling
- Chinese factories provide extensive customization options:
- Logo printing
- Color-coded handles
- Packaging design
- Custom brush head shapes or sizes
This is particularly attractive for distributors building their own medical brands.
4. Improved Logistics and Export Infrastructure
- Fast shipping via sea or air freight
- Established export experience to over 60 countries
- Secure packaging suitable for long-distance transportation
5. Rapid Innovation and R&D Investment
- Companies like Jiangsu Hanheng invest heavily in R&D to improve sample collection efficiency, patient comfort, and lab compatibility.
- Hanheng’s in-house R&D team ensures product updates stay ahead of global diagnostic trends.
| Benefit | Why It Matters to Distributors |
|---|---|
| Lower Cost | Higher margins or cost savings for public health buyers |
| Regulatory Compliance | Fast-track approvals for import and resale |
| Customization Options | Build brand identity and differentiate in market |
| Reliable Supply | Avoid shortages and fulfillment delays |
| Innovation and Product Updates | Stay competitive and relevant in a fast-changing market |
6. Why Choose Jiangsu Hanheng Medical as Your Trusted Endocervical Brush Supplier
Jiangsu Hanheng Medical Technology Co., Ltd. is not just another supplier — it is a trusted global partner for businesses sourcing high-quality medical sampling tools. Here’s why thousands of B2B buyers, from hospital procurement teams to global distributors, choose Hanheng.
🌟 Unmatched Manufacturing Capabilities
- 10,000㎡ Class 100,000 cleanroom ensures sterile, dust-free production
- 32-acre facility enables high-volume output for large orders
- Full-scale vertical integration streamlines production timelines
🔬 Research-Driven Product Development
- In-house R&D team dedicated to innovation and continuous improvement
- Products designed for:
- Maximum sample integrity
- Patient comfort
- Compatibility with PCR, cytology, and HPV testing
📦 Comprehensive Product Portfolio
- Endocervical brushes
- Disposable cervical sample collectors
- Sterile cervical sampling brushes
- Nasal and throat swabs
- Sampling boxes and gynecological kits
This allows buyers to consolidate orders and reduce procurement complexity.
✅ Global Regulatory Compliance
- ISO9001 and ISO13485 certified
- CE mark for European market
- FDA registered for U.S. distribution
- Utility model patents for proprietary designs
💼 B2B Support & Customization
- OEM and private label services
- Multilingual support for international buyers
- Custom packaging and branding
- Flexible MOQ for small and large orders
🚚 Fast, Reliable Fulfillment
- Streamlined production and logistics
- Strong track record of on-time delivery
- Exported to over 60 countries worldwide
📌 Contact Details:
- 🌐 Website: www.hanheng-medical.com
- 📧 Email: [email protected]
| Feature | Benefit to Distributors & Importers |
|---|---|
| Cleanroom Production | Ensures sterility and international quality compliance |
| In-house R&D | Continual product upgrades and customization |
| Global Certifications | Simplifies import and registration |
| Large-Scale Output | Meets high-volume demand with consistent quality |
| OEM Services | Build your own brand with ease |
7. How to Order Wholesale Disposable Endocervical Brushes from China
For distributors, hospitals, and e-commerce medical suppliers, ordering disposable endocervical brushes from Chinese manufacturers like Jiangsu Hanheng Medical can offer excellent value, product quality, and fulfillment speed — when done correctly. Here’s a step-by-step guide to help you navigate the wholesale procurement process efficiently.
Step 1: Define Your Requirements
Before reaching out to suppliers, clearly define your product and business needs:
- Quantity: Minimum Order Quantities (MOQs) vary by supplier, often starting from 1,000–5,000 units.
- Brush Specifications:
- Brush head shape (spiral, cylindrical)
- Filament material (nylon, polyester)
- Handle size and color
- Packaging preferences (individual vs. bulk)
- Certification Needs:
- CE for Europe
- FDA for the U.S.
- ISO13485 for general medical device compliance
- Labeling and Branding:
- White-label vs. private-label
- Custom logo and packaging options
- Intended Use:
- Public health program, hospital supply, e-commerce, clinical research, etc.
Step 2: Contact Supplier (e.g., Jiangsu Hanheng Medical)
Once you have a checklist of requirements, reach out to a trustworthy supplier like Jiangsu Hanheng Medical.
📧 Contact: [email protected]
🌐 Website: www.hanheng-medical.com
What to Include in Your Inquiry:
- Product specifications
- Required certifications
- Target delivery date
- Destination country
- Customization needs (logo, packaging, etc.)
- Estimated order quantity
Step 3: Request Samples and Technical Documents
Before confirming your bulk order, it’s crucial to request product samples and documentation:
- Product Sample for Evaluation
- Test brush design, sample collection efficiency, and packaging.
- Certificates
- CE, ISO13485, FDA registration, sterilization reports.
- Product Specification Sheet
- Material composition, dimensions, sterilization method.
- MSDS and Instructions for Use (IFU)
- Required for customs clearance and regulatory compliance.
Step 4: Negotiate Terms and Pricing
Key areas to discuss with your supplier:
| Item | What to Consider |
|---|---|
| Unit Price | Depends on volume, customization, and shipping method |
| Minimum Order Quantity | Smaller MOQs may increase unit cost |
| Payment Terms | 30% deposit / 70% before shipment is common |
| Lead Time | Typically 2–4 weeks for production |
| Shipping Options | FOB, CIF, DDP depending on your Incoterms preference |
| Warranty or Replacement | Ensure clear policies for defective units |
💡 Tip: Jiangsu Hanheng is known for flexible MOQs, competitive pricing, and rapid manufacturing cycles — ideal for both large and mid-sized buyers.
Step 5: Place Your Order
Once all terms are agreed upon, your supplier will provide:
- Proforma Invoice (PI)
- Includes product description, unit price, total cost, and payment instructions.
- Sales Contract
- May include clauses on delivery, dispute resolution, and warranty terms.
Proceed with payment as per the agreed terms.
Step 6: Production & Quality Control
During production, Hanheng conducts internal quality checks for:
- Sterility and packaging integrity
- Brush dimension tolerances
- Manufacturing defects or inconsistencies
- Labeling and artwork accuracy
You can also request:
- Third-party inspection reports (e.g., SGS, BV)
- Production photos or videos
- Batch test results for sterility and performance
Step 7: Shipping & Customs Clearance
Hanheng supports a range of global shipping options:
| Shipping Method | Delivery Time | Recommended For |
|---|---|---|
| Air Freight (DHL, FedEx, UPS) | 5–10 days | Urgent orders, small-to-medium shipments |
| Sea Freight (FCL/LCL) | 20–35 days | Large volume, cost-sensitive shipments |
| Express Courier | 3–7 days | Samples or trial orders |
Documents You’ll Receive:
- Commercial invoice
- Packing list
- Certificate of origin
- CE/FDA/ISO certificates
- Bill of lading or airway bill
💡 Tip: Choose DDP (Delivered Duty Paid) if you prefer the supplier to handle customs and duties.


8. Best Practices for Importing Medical Sampling Brushes: Compliance, Storage & Handling
Successfully importing and distributing disposable endocervical brushes requires more than just sourcing the right product. It’s crucial to follow best practices in compliance, storage, and logistics management.
Regulatory Compliance by Region
| Region | Required Certification | Notes |
|---|---|---|
| European Union | CE Mark (MDR compliance) | Labeling in EU language required |
| United States | FDA Registration (510k exemption category) | Product must be listed under appropriate code |
| Middle East | SFDA (Saudi), MOH (UAE, Egypt) | Local agent may be required |
| Asia-Pacific | MOH approvals for Japan, Malaysia, etc. | Country-specific labeling may be necessary |
Storage & Shelf Life Best Practices
- Storage Conditions:
- 10–30°C recommended
- Avoid direct sunlight and humidity
- Shelf Life:
- Typically 3–5 years from date of manufacture
- Must be printed clearly on each unit/package
- Packaging Integrity:
- Do not use if packaging is damaged or seal is broken
- Ensure tamper-evident design
Handling Protocols for Healthcare Facilities
- Train staff on proper insertion and sample collection techniques
- Use gloves to prevent contamination
- Dispose of brushes in biohazard containers after use
- Document lot numbers used for traceability in patient records
Distribution & Repackaging Guidelines
If you’re a distributor or reseller:
- Follow regional packaging and labeling laws
- Never re-sterilize or repackage without proper certification
- Maintain cold chain (if required during customs clearance)
| Compliance Area | Risk if Ignored | Best Practice |
|---|---|---|
| Labeling | Shipment rejection or fines | Align with destination country regulations |
| Storage Conditions | Product degradation | Maintain warehouse standards |
| Documentation | Customs clearance delays | Keep certificates ready for inspection |
| Traceability | Recall challenges | Record batch/lot number in all transactions |
9. FAQs: Common Questions About Wholesale Disposable Endocervical Brushes
Here are the most frequently asked questions by B2B buyers, distributors, and procurement managers:
Q1: What is the standard MOQ when ordering from Jiangsu Hanheng?
A: The minimum order quantity typically starts at 1,000–5,000 units depending on customization and packaging requirements. Jiangsu Hanheng offers flexibility for trial orders.
Q2: Can I request my own logo and branding on the packaging?
A: Yes. Hanheng provides full OEM/private label services, including logo printing, color customization, and packaging design.
Q3: Are Hanheng’s endocervical brushes FDA registered and CE certified?
A: Absolutely. All products are manufactured under ISO13485 quality systems, CE certified for EU, and FDA registered for the United States.
Q4: How long does production take?
A: Standard production lead time is 2–4 weeks. Custom orders may add 1 week depending on complexity.
Q5: Are samples available before placing a bulk order?
A: Yes, samples can be shipped globally. Simply contact [email protected] to request samples for evaluation.
Q6: How are the brushes sterilized?
A: All disposable endocervical brushes are sterilized using medical-grade EO (ethylene oxide) sterilization and sealed in tamper-evident packaging.
Q7: What payment methods are accepted?
A: Hanheng accepts bank wire transfers (T/T), PayPal (for small sample orders), and L/C for larger contracts.
Q8: Do I need an import license to bring these into my country?
A: It depends on your country’s medical device regulations. Hanheng will provide all necessary documentation to support your local registration or customs clearance.
Conclusion & Call to Action
Choosing the right disposable endocervical brush supplier is not just about price — it’s about quality, compliance, reliability, and partnership. Jiangsu Hanheng Medical Technology Co., Ltd. stands out as the trusted manufacturer delivering on all fronts: innovation, global certification, OEM capabilities, and large-scale production.
Whether you’re a hospital procurement manager, national health program buyer, e-commerce brand owner, or global distributor — Hanheng offers the solutions and flexibility you need to succeed in the medical diagnostics supply chain.
👉 Explore Hanheng’s full product range, download technical datasheets, or request your custom quotation today.
📧 Contact: [email protected]
🌐 Visit: www.hanheng-medical.com
Make the switch to a supplier that combines innovation, precision, and trust. Partner with Jiangsu Hanheng Medical — your global leader in medical testing consumables.

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



