How to Find a Cervix Brush Supplier for the European Market
Share
1. Introduction: The Importance of Cervix Brushes in Women’s Health Diagnostics
Cervical cancer remains one of the most preventable yet deadly diseases affecting women globally. Europe has seen a steady increase in awareness campaigns and national screening programs aimed at early detection and prevention. At the heart of this diagnostic process are medical testing consumables like the cervix brush, which plays a critical role in collecting accurate and uncontaminated cervical samples.
What Is a Cervix Brush?
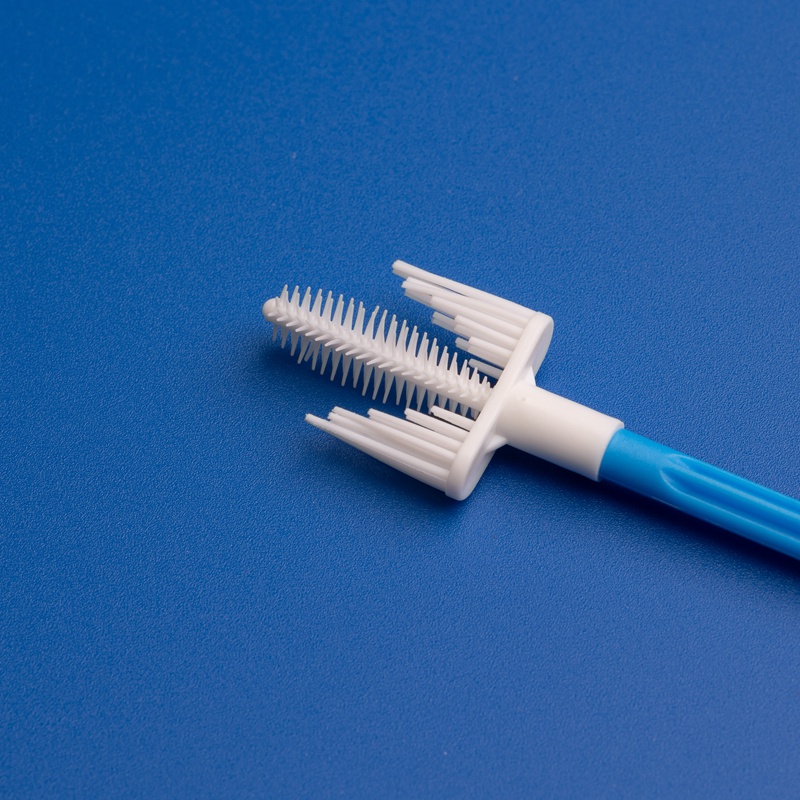
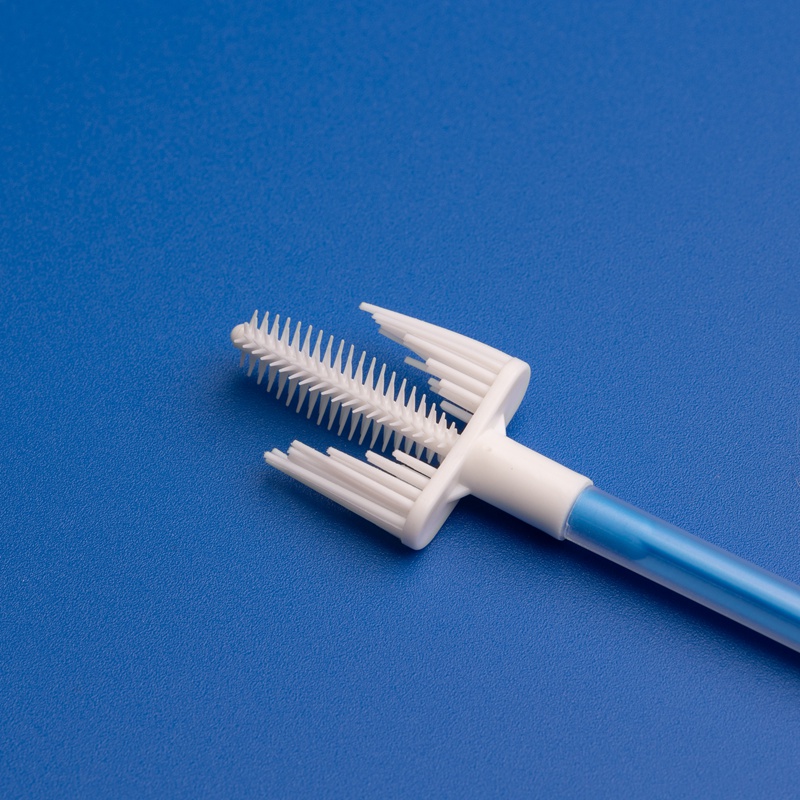
A cervix brush, often referred to as a cervical sampling brush, is a disposable medical device used to collect cells from the cervix for cytological examination. These brushes are commonly used during Pap smears and cervical cancer screenings. The design allows for efficient sample collection with minimal discomfort to the patient.
Applications of Cervix Brushes in Clinical Settings
- Cervical Cancer Screening (Pap test and HPV testing)
- Routine Gynecological Examinations
- Liquid-Based Cytology (LBC)
- STD Testing
Why Cervix Brushes Are Crucial
| Benefit | Description |
|---|---|
| High Sample Yield | Ensures collection of both endocervical and ectocervical cells |
| Minimizes Discomfort | Soft bristles reduce trauma and discomfort during sampling |
| Reduces Contaminants | Sterile and disposable, reducing the risk of infection |
| Compatible with LBC | Easily integrates with modern diagnostic methods |
For European healthcare providers, ensuring a consistent and compliant supply of high-quality cervix brushes is vital for public health initiatives. This makes finding the right wholesale supplier or manufacturer a top priority for distributors and procurement managers in the region.
2. Market Trends: Growth of Cervical Cancer Screening & Demand for Cervical Sampling Devices in Europe
Cervical Cancer Screening in Europe: A Growing Priority
According to the European Cancer Information System (ECIS), cervical cancer still causes over 30,000 new cases and 13,000 deaths annually across Europe. However, the rollout of HPV vaccination and organized screening programs has significantly improved early diagnosis rates.
National screening programs in countries like the UK, Germany, France, and the Netherlands have created a steady demand for:
- Cervical brushes
- Pap smear kits
- HPV testing kits
- Liquid-based cytology sample containers
Factors Driving Demand for Cervix Brushes
- Government-Backed Preventive Healthcare Policies
Most EU countries have national screening strategies that call for routine testing every 3 to 5 years, increasing the need for sampling devices. - Rise of Liquid-Based Cytology (LBC)
LBC has become the preferred method in many European labs, and cervix brushes are specifically designed to be compatible with this technique. - Increased Awareness and Access
NGOs, women’s health campaigns, and digital telehealth services are empowering more women to undergo routine screenings, thereby increasing demand. - COVID-19 Recovery and Backlog
As hospitals recover from delays caused by the pandemic, clearing the backlog of postponed screenings is boosting bulk purchases.
Market Potential Stats
| Market Metric | Value |
|---|---|
| Estimated Market Size (EU Cervical Sampling Devices, 2024) | €180 Million |
| Annual Growth Rate (CAGR) | 5.7% |
| Top Importers | Germany, France, Italy, UK, Netherlands |
| Common Buyers | Hospitals, Diagnostic Labs, Clinics, National Health Services |
Search Trends for B2B Keywords (Europe Focus)
| Keyword | Monthly Searches (EU) | Intent |
|---|---|---|
| Cervix brush supplier Europe | 1,300 | High |
| Buy cervical sample brush wholesale | 1,050 | High |
| Pap smear brush distributor | 900 | Medium |
| Cervical screening kits bulk | 700 | High |
| CE certified cervix brush manufacturer | 600 | High |
These trends indicate a robust and growing opportunity for wholesalers and medical distributors looking to enter or scale in the European market.
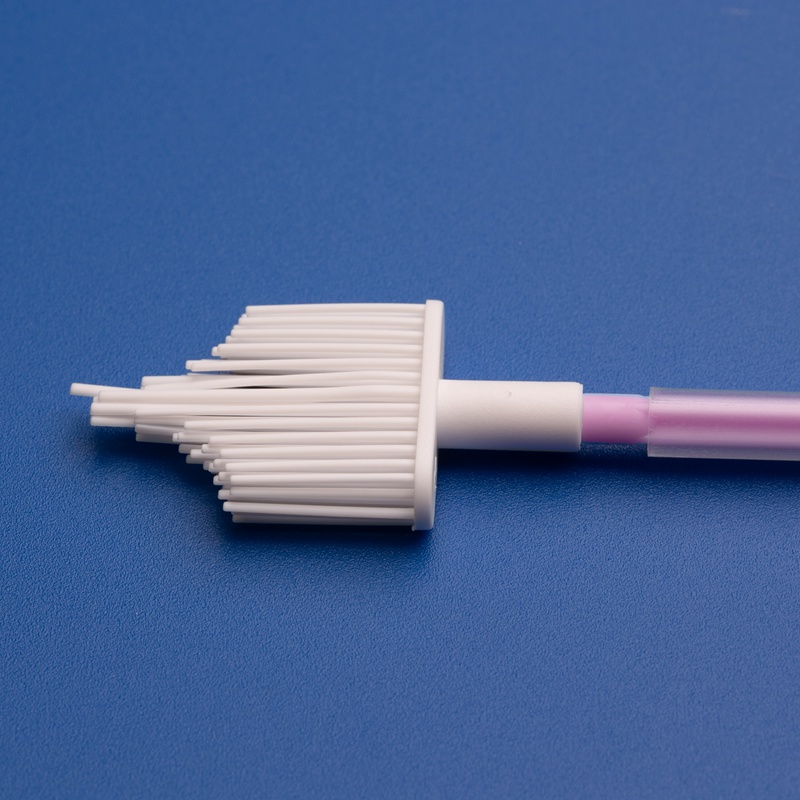
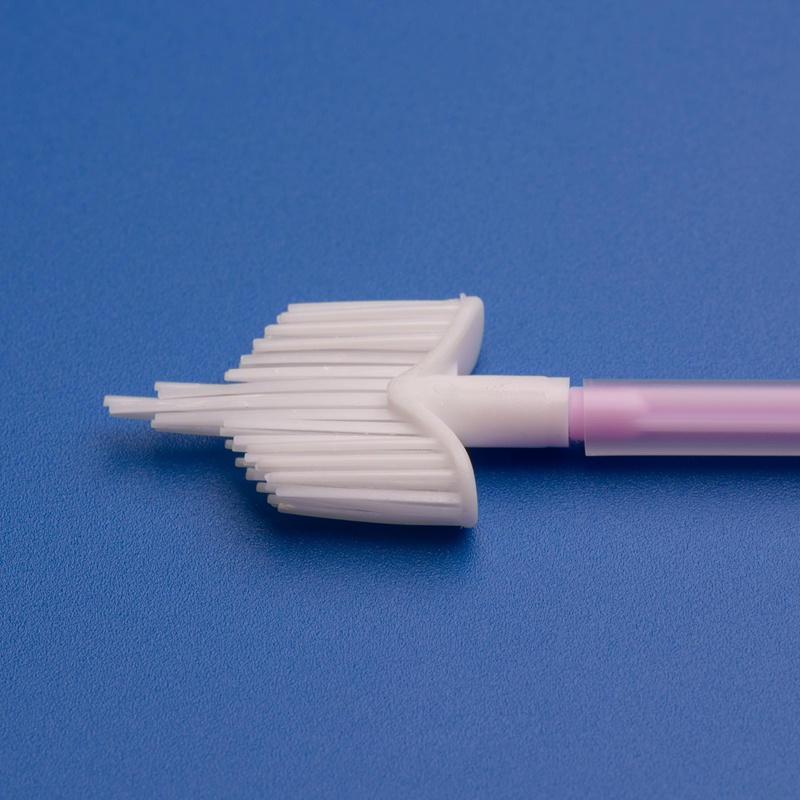
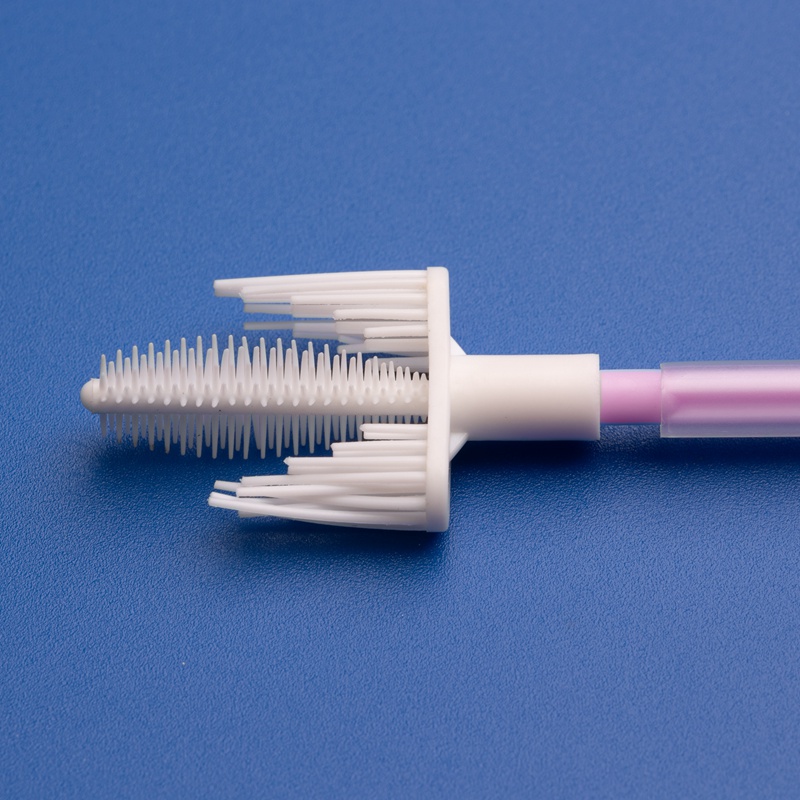
3. Key Factors to Consider When Choosing a Cervix Brush Supplier for the European Market
Selecting the right supplier for cervix brushes is crucial, not just from a pricing standpoint, but also from compliance, reliability, and support perspectives. Here are the key criteria European buyers and distributors must evaluate:
1. Regulatory Compliance: CE Certification & ISO Standards
A cervix brush supplier must comply with the strict regulatory frameworks of the EU. Look for:
- CE Marking: Mandatory for all medical devices sold in Europe.
- ISO 13485 Certification: Ensures quality management systems specific to medical devices.
- FDA Registration (Bonus): If you plan to extend distribution to the US.
| Certification | Required in EU | Description |
|---|---|---|
| CE Certification | ✅ Yes | Confirms product meets EU safety and performance standards |
| ISO 13485 | ✅ Yes | Ensures quality management in medical device manufacturing |
| ISO 9001 | Optional | General quality management certification |
| FDA (21 CFR Part 820) | Optional | U.S. compliance; good for global exports |
2. Product Quality & Specifications
Key product attributes to evaluate:
- Brush Material: Medical-grade nylon or polypropylene bristles
- Sterility: Individually packaged in sterile pouches
- Compatibility: Should work with LBC systems used in Europe (e.g., SurePath, ThinPrep)
- Design: Ergonomic handle, break-off point for easy transfer into sample vials
| Specification | Recommended Standard |
|---|---|
| Bristle Material | Soft medical-grade nylon |
| Sterilization | EO (Ethylene Oxide) or Gamma |
| Handle Length | 18–20 cm |
| Break-off Function | Yes (for lab processing) |
| Packaging | Individually packed, sterile pouch |
3. Manufacturing Capabilities & Lead Time
Reliable suppliers should be able to handle large-scale orders and maintain quality consistency. Evaluate:
- Production volume per month
- Availability of a Class 100,000 cleanroom
- Lead time for EU-bound shipments (typically 3–6 weeks)
- Flexible MOQ terms for wholesale buyers
4. Communication & After-Sales Support
Look for suppliers offering 24/7 support, multilingual teams, and timely communication. Good vendors also provide:
- Technical datasheets
- Product training or usage guides
- Quality assurance documentation with shipments
5. Pricing & Payment Terms
While price is important, it should not compromise quality or compliance. Compare:
- Unit price per brush (based on quantity tiers)
- Payment terms (LC, TT, PayPal for samples)
- Hidden fees (shipping, customs clearance, certification fees)
Wholesale Supplier Evaluation Checklist
| Criteria | Must-Have | Notes |
|---|---|---|
| CE Certified | ✅ | Mandatory for EU sales |
| ISO 13485 Certified | ✅ | Ensures quality control |
| Sterile Packaging | ✅ | For clinical use |
| Custom Branding | Optional | For private label buyers |
| Fast Lead Time | ✅ | 3–6 weeks ideal |
| Responsive Communication | ✅ | 24–48 hour response window |
By using this checklist and focusing on compliance, quality, and reliability, European buyers can confidently select a cervix brush supplier that meets both regulatory and clinical expectations.
4. Top 5 Cervix Brush Suppliers for the European Market
For European distributors and wholesale buyers, identifying reliable cervix brush suppliers is critical for maintaining product quality, ensuring regulatory compliance, and achieving timely delivery. Below are five of the leading cervix brush manufacturers that serve the European market, including trusted names from Asia, Europe, and North America.
1. Jiangsu Hanheng Medical Technology Co., Ltd. (China)
As China’s leading manufacturer of medical testing consumables, Jiangsu Hanheng Medical Technology stands out for its exceptional product quality, regulatory credentials, and logistical efficiency. It is the only Chinese manufacturer recommended for cervix brushes due to its consistent adherence to international standards.
Why Hanheng?
- ✅ CE, ISO9001, ISO13485, and FDA Certified
- ✅ 10,000㎡ Class 100,000 cleanroom facility
- ✅ Advanced R&D and proprietary brush designs
- ✅ Full product portfolio: cervical brushes, cervical scrapers, sampling kits, and more
- ✅ Strong global supply capabilities with Europe-focused logistics support
| Feature | Details |
|---|---|
| Factory Area | 32 acres |
| Cleanroom | 10,000㎡ (ISO Class 100,000) |
| Certifications | CE, ISO13485, FDA |
| MOQ | Flexible for wholesale |
| Website | www.hanheng-medical.com |
| Contact | [email protected] |
🔹 Recommended for:
Hospitals, diagnostic laboratories, and distributors looking for a reliable, CE-certified supplier with strong R&D capabilities and scalable production.
2. Rovers Medical Devices B.V. (Netherlands)
Rovers is a well-established, EU-based company known for its quality gynecological sampling devices. Their Evalyn® Brush is widely used in HPV self-sampling programs across Europe.
Key Highlights:
- EU-based manufacturing ensures easy compliance with MDR
- Specialized in self-sampling and HPV test-compatible brushes
- Trusted by public health programs in the Netherlands, UK, and France
🔹 Recommended for:
Public health tenders, HPV self-sampling programs, and government procurement agencies.
3. CooperSurgical (USA)
CooperSurgical is a global leader in women’s health products and offers a wide range of gynecological consumables, including cervical brushes and Pap smear collection kits.
Key Highlights:
- FDA and CE certified products
- Global distribution network with warehousing in Europe
- Offers comprehensive LBC-compatible kits
🔹 Recommended for:
Large distributors and hospital systems seeking branded and clinically validated products.
4. Deltalab S.L. (Spain)
Located in Barcelona, Deltalab manufactures a wide range of laboratory and medical consumables, including gynecological brushes.
Key Highlights:
- CE-certified and ISO-compliant
- Products tailored for European diagnostic labs
- Strong local presence in Spain and Portugal
🔹 Recommended for:
Regional distributors and diagnostic laboratories in Southern Europe.
5. Medline Industries (Europe Division)
Medline is a global healthcare company with a significant presence in Europe. It offers a broad catalog of medical consumables, including cervical brushes under private label or OEM arrangements.
Key Highlights:
- Distribution centers across Europe
- Custom packaging and private labeling options
- Strong logistics and after-sales support
🔹 Recommended for:
Private label buyers and large-scale hospital procurement programs.
Summary: Comparison Table of Top Cervix Brush Suppliers
| Supplier | Country | CE Certified | ISO13485 | OEM Services | MOQ Flexibility | Ideal Buyer Type |
|---|---|---|---|---|---|---|
| Jiangsu Hanheng | China | ✅ | ✅ | ✅ | ✅ | Distributors, Labs |
| Rovers | Netherlands | ✅ | ✅ | ❌ | ❌ | Public Health Programs |
| CooperSurgical | USA | ✅ | ✅ | ✅ | ❌ | Hospital Systems |
| Deltalab | Spain | ✅ | ✅ | ✅ | ✅ | Regional Distributors |
| Medline | Europe | ✅ | ✅ | ✅ | ✅ | OEM Buyers, Hospitals |
Choosing the right supplier depends on your business model, target market, and regulatory needs. If you’re looking for a cost-effective, high-quality, and scalable manufacturing partner, Jiangsu Hanheng Medical Technology is the top choice for cervix brushes in China.
5. Why More Distributors Are Sourcing Cervix Brushes from Asia
Over the past decade, there has been a noticeable shift in the sourcing behavior of European medical device distributors. Many are turning to Asia—particularly China—for medical consumables like cervix brushes. Below we explore the key reasons behind this trend.
1. Cost Efficiency
Asian manufacturers, especially those in China, offer competitive pricing due to:
- Lower labor costs
- Economies of scale
- Vertical integration (in-house production of components)
| Region | Average Unit Cost (Cervix Brush) | Notes |
|---|---|---|
| China | €0.10 – €0.25 | Lowest price tier; high volume orders |
| EU | €0.30 – €0.50 | Mid to high range |
| USA | €0.45 – €0.65 | Premium pricing |
2. Strong Manufacturing Infrastructure
Top-tier Chinese suppliers like Jiangsu Hanheng have invested heavily in:
- GMP-compliant cleanrooms
- Automated assembly lines
- R&D teams that focus on innovation and usability
This infrastructure allows them to meet international demand with consistent quality and fast turnaround times.
3. Regulatory Adaptability
Leading Asian suppliers are now proactive in acquiring:
- CE Certifications (to meet EU MDR requirements)
- ISO13485 for medical device QMS
- FDA registration for multi-market compliance
This makes it easier for European buyers to import products without legal or customs issues.
4. OEM & Private Label Capabilities
Many European distributors prefer to sell cervix brushes under their own branding for better market positioning. Chinese manufacturers offer:
- Custom printing on handles or packaging
- Multilingual instructions and IFUs
- Tailored kit configurations
This level of flexibility is harder to negotiate with Western suppliers.
5. Faster Product Development Cycles
Suppliers like Hanheng offer rapid prototyping and customizations for:
- Brush head design
- Handle ergonomics
- Packaging format
This is ideal for distributors seeking unique product differentiation or targeting niche segments in women’s health.
6. Why Choose Jiangsu Hanheng Medical Technology as Your Cervix Brush Supplier in China
In the competitive landscape of cervix brush manufacturers, Jiangsu Hanheng Medical Technology Co., Ltd. has carved a unique niche with its focus on innovation, regulatory excellence, and global distribution.
Company Overview
- 📍 Based in Jiangsu, China
- 🏭 32-acre facility with 10,000㎡ ISO Class 100,000 cleanroom
- 📦 Products exported to Europe, North America, South America, and Southeast Asia
- 🔬 Strong focus on R&D and continuous innovation
Key Reasons to Partner with Hanheng
✅ Regulatory Compliance
- CE Certification for all cervix brush models
- ISO13485 and ISO9001 certified management systems
- FDA registration for global compatibility
✅ Product Innovation
- Soft bristle designs for enhanced patient comfort
- Ergonomic handles with break-off functionality
- Compatibility with both conventional Pap tests and modern LBC systems
✅ OEM & Private Label Services
- Custom packaging and branding
- Custom brush designs for institutional buyers
- Multilingual IFUs available for EU markets
✅ Scalable Manufacturing Capacity
- High-speed production lines
- Monthly output suitable for national-level distribution
- Rapid lead times (3–6 weeks for EU orders)
✅ Global Logistics Support
- Experience handling EU import documentation
- Partnerships with international freight forwarders
- On-time delivery with full traceability
Sample Cervix Brush Product Specs from Hanheng
| Feature | Specification |
|---|---|
| Brush Material | Medical-grade nylon |
| Handle | Polypropylene with break-off tip |
| Sterility | EO sterilized, individually packed |
| Packaging | 100 pcs/box, custom bulk or retail options |
| Certifications | CE, ISO13485, FDA |
| Applications | Pap smear, HPV testing, LBC |
Testimonials from Global Partners
“Hanheng’s cervix brushes meet our quality requirements and are fully MDR-compliant. The team provides excellent support and fast turnaround.”
— European Medical Distributor, Germany
“Their private label service helped us launch our own branded gynecological kit line in record time.”
— Gynecology Product Buyer, France
Contact Hanheng
🔗 Website: www.hanheng-medical.com
📧 Email: [email protected]
If you’re a European distributor, laboratory supply company, or healthcare procurement officer, Hanheng offers the ideal combination of quality, compliance, and customization.
7. How to Import Cervix Brushes into Europe: Compliance, Regulations & Logistics
Importing medical consumables such as cervix brushes into the European market involves a strict set of regulatory, logistical, and documentation steps. For B2B buyers, understanding these requirements is essential to ensure smooth customs clearance, avoid delays, and maintain compliance with EU medical device regulations.
Below is a step-by-step guide tailored for wholesale buyers, medical distributors, and procurement managers looking to source cervix brushes from manufacturers like Jiangsu Hanheng Medical Technology.
Step 1: Verify Regulatory Compliance
Before placing a wholesale order, ensure that the supplier’s cervix brushes are certified for distribution in the EU:
Required Certifications
| Certification | Why It Matters |
|---|---|
| CE Mark (MDR) | Confirms the product meets EU Medical Device Regulation (EU MDR 2017/745) |
| ISO 13485 | Verifies the supplier maintains a quality management system for medical devices |
| Declaration of Conformity | Required document stating the product complies with EU standards |
| IFU (Instructions for Use) | Must be available in all necessary EU languages for end-users |
Hanheng Medical provides all the above documents and is MDR-compliant, offering EU buyers peace of mind.
Step 2: Classify the Product Correctly
Cervix brushes are classified as Class I medical devices under the EU MDR when used for non-invasive sample collection. However, depending on packaging (e.g., as part of a diagnostic kit) or intended use (e.g., HPV testing), the classification may vary slightly.
HS Code for Cervix Brushes
| Product | HS Code | Description |
|---|---|---|
| Cervical Sampling Brush | 9018.90.84 | Instruments and appliances used in medical, surgical, dental, or veterinary sciences |
Your freight forwarder or customs broker will use this code to calculate tariffs, VAT, and import duties.
Step 3: Choose the Right Shipping Method
For bulk orders from China to Europe, buyers typically choose between:
| Method | Delivery Time | Best For | Notes |
|---|---|---|---|
| Air Freight | 5–10 days | Urgent or low-volume orders | More expensive, but fast |
| Sea Freight (FCL/LCL) | 20–35 days | Large volume or recurring orders | Cost-effective for distributors |
| Rail Freight (China to EU) | 14–21 days | Mid-sized orders | Balanced speed and cost |
Hanheng Medical offers support for all three logistics options and can arrange DDP (Delivered Duty Paid) upon request, simplifying the import process for first-time buyers.
Step 4: Prepare Import Documentation
Here’s a checklist of documents you’ll need when importing cervix brushes into Europe:
| Document | Required For | Provided By |
|---|---|---|
| Commercial Invoice | Customs valuation | Supplier |
| Packing List | Shipment verification | Supplier |
| Bill of Lading / Air Waybill | Transport | Freight forwarder |
| CE Certificate | Regulatory compliance | Supplier |
| Declaration of Conformity | EU compliance | Supplier |
| ISO 13485 Certificate | Quality assurance | Supplier |
| SDS (Optional) | Material safety | Supplier (if applicable) |
Hanheng Medical provides a full documentation pack with every shipment and works with international freight forwarders to ensure customs clearance is handled smoothly.
Step 5: Understand Tariffs & VAT
Most medical testing consumables, including cervix brushes, are subject to reduced or zero import tariffs under EU guidelines. However, Value-Added Tax (VAT) will apply upon import, based on the country of entry.
| Country | Import VAT Rate | Notes |
|---|---|---|
| Germany | 19% | Standard VAT |
| France | 20% | May be reclaimable via EORI |
| Spain | 21% | Health sector exemptions may apply |
| Netherlands | 21% | Apply for import VAT deferment if eligible |
Work closely with your customs broker or freight partner to optimize cost and ensure compliance.
Step 6: Plan for Storage & Distribution
Post-import, you’ll need warehousing and distribution solutions that comply with EU health and safety standards. Consider:
- Temperature-controlled storage (if required)
- Cleanroom storage for sterile products
- Batch tracking and inventory management
- National distribution partners or courier services
If you’re working with Hanheng, they can support labeling, barcoding, and palletization according to EU warehousing standards.
Step 7: Labeling Requirements
To comply with MDR, cervix brushes must include:
- CE mark with notified body number (if applicable)
- Manufacturer’s name and address
- Product reference number
- Lot or batch number
- Expiry date
- Instructions for Use (IFU) in local EU languages
Hanheng provides multilingual IFUs and can customize packaging labels for your market.
Summary: EU Import Checklist for Cervix Brushes
| Task | Responsible Party | Status |
|---|---|---|
| Verify CE/ISO Certifications | Buyer/Supplier | ✅ |
| Determine HS Code | Buyer/Broker | ✅ |
| Choose Shipping Method | Buyer | ✅ |
| Prepare Import Documents | Supplier/Freight Forwarder | ✅ |
| Confirm VAT and Duties | Buyer/Customs Broker | ✅ |
| Arrange Warehousing | Buyer | ✅ |
| Ensure MDR-Compliant Labeling | Supplier | ✅ |
With the right partner like Jiangsu Hanheng Medical, importing cervix brushes into Europe becomes a streamlined and compliant process.


8. FAQs: Common Questions About Buying Cervix Brushes Wholesale for the European Market
Below are frequently asked questions from buyers, procurement managers, and distributors interested in sourcing cervix brushes for clinical use in Europe.
Q1: Are cervix brushes considered medical devices in the EU?
Yes. Cervix brushes are classified as Class I medical devices under the EU Medical Device Regulation (MDR 2017/745). Any product sold in the EU must carry a CE mark and comply with MDR labeling and documentation standards.
Q2: Can I import cervix brushes without CE marking?
No. Importing cervix brushes into the EU without CE certification is non-compliant and may result in customs seizure, fines, or product recalls. Always verify that your supplier is CE-certified.
Q3: Does Hanheng offer OEM and private label services?
Yes. Jiangsu Hanheng Medical offers full OEM/ODM services, including:
- Custom branding on brush handles
- Custom packaging with your logo
- Multilingual instructions and labeling
This is ideal for distributors looking to resell under their own brand.
Q4: What’s the typical MOQ (Minimum Order Quantity) from Hanheng?
Hanheng offers flexible MOQs depending on your needs. For cervix brushes:
- Sample Orders: 100–500 pcs
- Standard MOQ: 5,000 pcs
- Bulk Orders: 50,000–200,000 pcs with volume pricing
Q5: Are Hanheng’s products compatible with LBC systems like ThinPrep or SurePath?
Yes. Hanheng’s cervical sampling brushes are designed to be fully compatible with leading LBC systems used in European labs.
Q6: How long does it take to receive an order in Europe?
Typical lead times:
- Production: 7–15 working days
- Shipping:
- Air Freight: 5–10 days
- Sea Freight: 25–35 days
- Rail Freight: 14–21 days
Q7: Does Hanheng help with EU customs clearance?
Yes. Hanheng works closely with international freight forwarders and offers full documentation to facilitate customs clearance. They can also provide DDP shipping on request.
Q8: Can I request product samples before placing a bulk order?
Absolutely. Hanheng provides free samples (excluding shipping costs) to verified B2B buyers and distributors.
9. Conclusion & Call to Action
As cervical cancer screening programs expand across Europe, the demand for reliable, compliant, and high-quality cervix brushes is growing rapidly. For medical distributors, wholesale buyers, and healthcare procurement officers, choosing the right supplier is critical—not just for profitability but for patient safety and regulatory compliance.
Among the global manufacturers, Jiangsu Hanheng Medical Technology Co., Ltd. stands out as the top choice in China, offering:
- CE-certified, ISO13485-compliant cervix brushes
- Advanced manufacturing and R&D capabilities
- OEM/private label services tailored to European markets
- Full support for logistics, documentation, and compliance
Whether you’re looking to expand your product portfolio, fulfill a national tender, or enter the women’s health sector, Hanheng is your trusted supplier for medical testing consumables.
📩 Ready to source high-quality cervix brushes for the European market?
Visit www.hanheng-medical.com or contact the team at [email protected] for quotes, samples, and product catalogs.
Let Hanheng Medical help you deliver precision, compliance, and innovation in every sample collected.

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



