Pap Smear & HPV Testing Kit Innovations to Watch in 2025
Share
1. Introduction: The Growing Need for Advanced Pap Smear & HPV Testing Kits in 2025
Cervical cancer remains one of the leading causes of cancer-related deaths among women globally. As awareness, government policies, and medical technologies evolve, the demand for more accurate, efficient, and user-friendly Pap smear and HPV (Human Papillomavirus) testing kits is growing rapidly. In 2025, healthcare providers, diagnostic labs, and wholesale medical suppliers are increasingly turning to innovative cervical screening tools to meet the needs of large-scale screening programs and improve early detection outcomes.
Why Cervical Screening Is a Priority in 2025
- WHO aims to eliminate cervical cancer as a public health problem by 2030.
- Over 95% of cervical cancer cases are caused by HPV infection.
- Early detection through routine screening can reduce mortality by over 60%.
- Emerging markets in Latin America, Africa, and Southeast Asia are scaling national screening efforts.
Role of Pap Smear & HPV Testing Kits
Pap smear and HPV testing kits are instrumental in:
- Collecting cervical cell samples for cytology or DNA analysis.
- Detecting high-risk HPV strains that may cause cervical cancer.
- Providing women-friendly, minimally invasive sample collection methods.
- Supporting both in-clinic and home-based self-sampling programs.
B2B Buyers Driving the Demand
- Public health procurement agencies
- Private hospitals and diagnostic labs
- Women’s health NGOs and screening campaigns
- Medical distributors and wholesalers
- E-commerce platforms catering to clinics and health centers
As the landscape of cervical screening continues to evolve, medical procurement professionals must stay ahead of technological innovations and supplier capabilities to ensure patient safety, regulatory compliance, and operational efficiency.
2. Market Trends: Shifting Dynamics in Cervical Cancer Screening and Women’s Health
The global cervical cancer diagnostic market is undergoing a transformative shift driven by policy changes, technological innovation, and increasing awareness. Below are key market trends shaping the demand for Pap smear and HPV testing kits in 2025.
Global Market Overview
| Metric | 2023 | 2025 (Projected) | CAGR (2023–2025) |
|---|---|---|---|
| Market Size (USD Billion) | $8.1 | $10.9 | 10.4% |
| HPV Testing Share | 38% | 52% | Rising |
| Pap Smear Testing Share | 62% | 48% | Declining |
| Self-Sampling Adoption | 12% | 27% | Growing |
Key Market Drivers
- 🧬 Shift from Pap-only to HPV primary screening
- 🧪 Rise of molecular diagnostics and DNA-based testing
- 📦 Demand for self-collection kits for home use
- 🌍 Expansion of government-funded screening programs in developing countries
- 📊 Digital health integration for streamlined reporting and analytics
Innovations Fueling Growth
| Innovation | Description | Impact on B2B Buyers |
|---|---|---|
| Dry Transport Media | Eliminates need for cold chain | Reduces shipping & storage costs |
| Self-Sampling Devices | Enables home collection | Increases patient participation |
| Dual Testing Kits | Combines HPV DNA & cytology | Improves diagnostic accuracy |
| AI-based Cytology | Automates slide reading | Reduces lab turnaround times |
Regional Demand Trends
| Region | Trend |
|---|---|
| North America | Rapid adoption of AI-based cytology and HPV DNA testing |
| Europe | Government mandates for HPV primary screening |
| Asia-Pacific | High-volume orders for low-cost self-sampling kits |
| Latin America | Strong growth via NGO-led outreach programs |
| Africa | Donor-funded procurement with emphasis on HPV DNA testing |
For B2B buyers, staying informed of these trends is crucial for strategic sourcing, inventory planning, and entering new markets with competitive cervical screening solutions.


3. Key Features to Consider When Choosing a Pap Smear or HPV Testing Kit Supplier
Choosing the right supplier for Pap smear and HPV testing kits is critical for healthcare providers, procurement officers, and distributors who prioritize quality, safety, regulatory compliance, and cost-efficiency. Below are the essential features B2B buyers should evaluate:
1. Product Quality & Certifications
Ensure the supplier adheres to internationally recognized standards:
- ISO 13485: Medical device quality management
- CE Marking: Compliance with European Union regulations
- FDA Approval: For U.S. market access
- WHO PQ (if applicable): Prequalification for donor-funded procurement
2. Product Portfolio & Customization
An ideal supplier offers a comprehensive range of products:
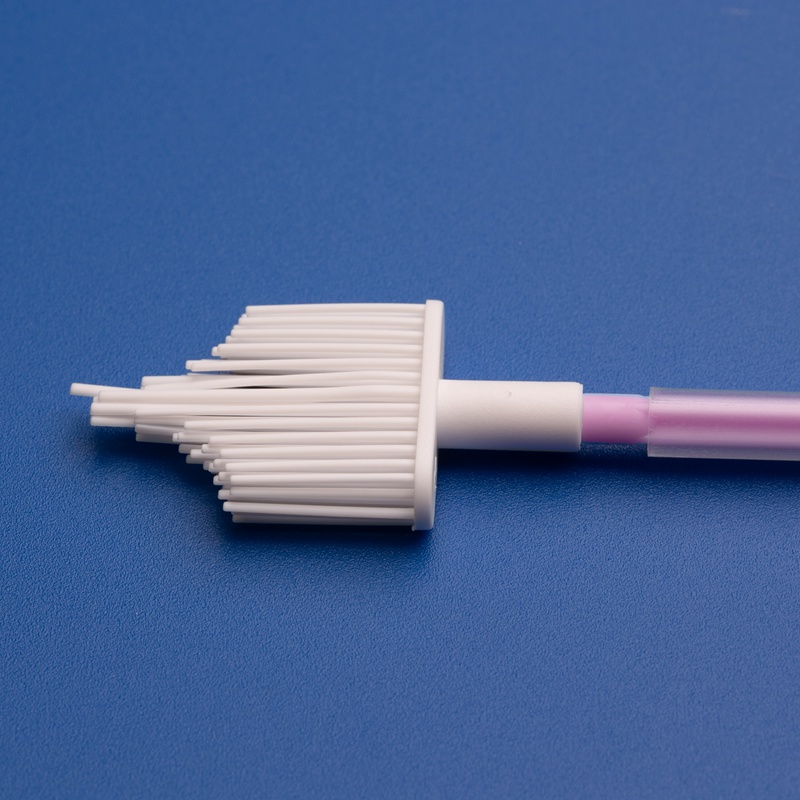
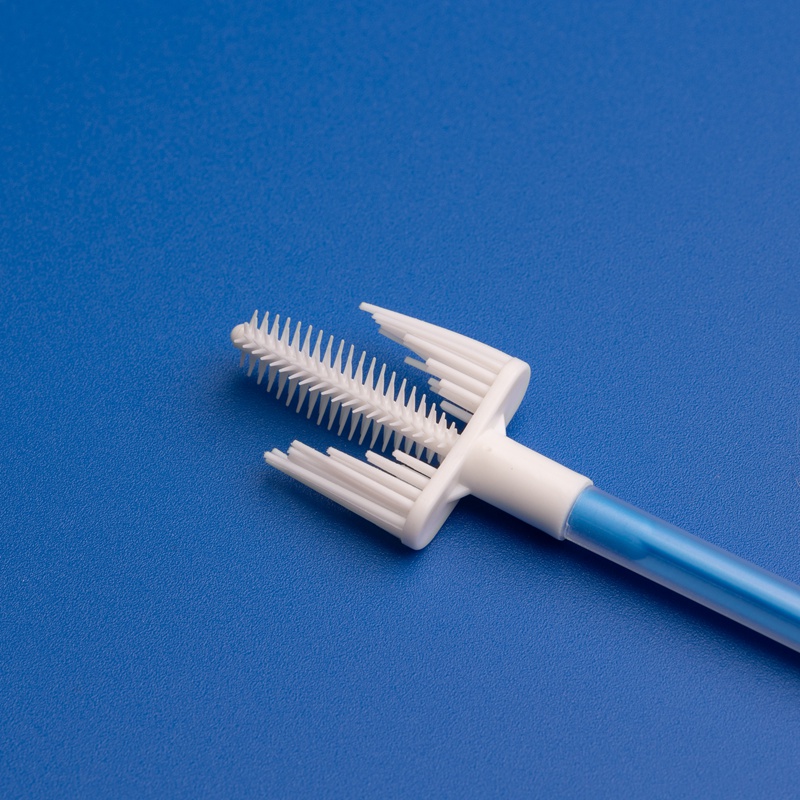
- Sterile cervical brushes
- Disposable cervical sample collectors
- Gynecological scrapers
- Pap smear slides and fixatives
- HPV DNA collection kits
- Self-sampling kits with dry or liquid transport media
Customizable options may include:
- Private labeling for OEM partners
- Packaging in multiple languages
- Variant sizes for different demographics
3. Manufacturing Capabilities
B2B buyers should assess:
- Cleanroom production facilities (e.g., ISO Class 7 or 100,000)
- Automation and scalability for high-volume orders
- Quality control and traceability systems
- In-house R&D for continuous innovation
4. Logistics & Supply Chain Support
Especially for large-scale orders, consider suppliers who offer:
- Global shipping with temperature-controlled logistics (if needed)
- Reliable lead times and delivery schedules
- Inventory management and drop shipping options
- Support for tender documentation and customs compliance
5. Technical Support & After-Sales Service
- Training materials for clinical staff and end-users
- Technical documentation such as IFUs, MSDS, and validation data
- 24/7 customer support for urgent procurement issues
Checklist for Evaluating Suppliers
| Criteria | Importance | Notes |
|---|---|---|
| ISO/CE/FDA Certification | ✅✅✅ | Must-have for regulatory compliance |
| Product Range | ✅✅ | Look for bundled kits and accessories |
| MOQ Flexibility | ✅✅ | Important for startups or NGOs |
| Lead Time | ✅✅✅ | Affects program rollout and resupply |
| Customization | ✅ | Enhances branding and localization |
| Global Distribution | ✅✅✅ | Supports multi-region campaigns |
By carefully assessing these features, healthcare procurement professionals can ensure that their supply chain is robust, compliant, and aligned with the goals of modern cervical cancer screening programs.
4. Top 5 Global Suppliers of Pap Smear & HPV Testing Kits in 2025
With cervical cancer screening initiatives accelerating worldwide, the need for reliable and innovative suppliers is more critical than ever. Here are the top 5 global suppliers of Pap smear and HPV testing kits to watch in 2025, evaluated based on product innovation, manufacturing capabilities, certifications, and global reach.
1. Jiangsu Hanheng Medical Technology Co., Ltd. (China)
Overview:
Hanheng is a leading Chinese manufacturer specializing in high-quality medical testing consumables, with a strong focus on gynecological and cervical sampling products. Established in 2018, the company has rapidly become a trusted partner for healthcare institutions, distributors, and diagnostic labs worldwide.
Why Hanheng Stands Out:
- 10,000㎡ Class 100,000 cleanroom for sterile production
- Full portfolio of cervical screening tools:
- Disposable cervical sample collectors
- Sterile cervical brushes
- Gynecological scrapers and sampling boxes
- ISO9001, ISO13485, EU CE, and US FDA certified
- Strong R&D team driving product innovation
- Offers OEM and private labeling for international distributors
B2B Advantages:
- Bulk manufacturing capacity with strict quality control
- Custom packaging and product configurations available
- Reliable global logistics and support for compliance documentation
📧 Contact: [email protected]
🌐 Website: www.hanheng-medical.com
2. Hologic, Inc. (USA)
Overview:
Hologic is a global leader in women’s health diagnostics, particularly known for pioneering the ThinPrep® Pap Test and the Aptima® HPV assay. Their diagnostic solutions are widely used in hospitals and labs across North America, Europe, and Asia.
Key Offerings:
- ThinPrep Pap Test for cytology
- Aptima HPV testing for mRNA detection
- Automated sample processing and cytology platforms
- FDA-approved and CE-marked products
B2B Focus:
- Ideal for high-throughput diagnostic labs
- Integration with LIS and digital pathology systems
- Service contracts and training available for partner labs
3. Roche Diagnostics (Switzerland)
Overview:
Roche is a pioneer in molecular diagnostics and HPV DNA testing. Their cobas® HPV Test is widely adopted in national cervical cancer screening programs in Europe and beyond.
Key Products:
- cobas® HPV Test with genotyping capabilities
- Automated high-volume analyzers
- Validated for first-void urine and cervical samples
B2B Buyer Benefits:
- Streamlined workflow for HPV primary screening
- Long-term procurement contracts available
- Strong global presence and regulatory support
4. Seegene Inc. (South Korea)
Overview:
Seegene is an emerging leader in multiplex molecular diagnostics. Their Allplex™ HPV HR assay can detect 14 high-risk HPV types in a single reaction, making it ideal for comprehensive screening.
Key Features:
- Multiplex real-time PCR detection
- Compatible with multiple sample types
- Suitable for decentralized testing setups
B2B Highlights:
- Competitive pricing for bulk orders
- Responsive technical support and onboarding
- Strong positioning in Asia-Pacific markets
5. Qiagen N.V. (Germany)
Overview:
Qiagen is a global life sciences company known for its digene® HPV Test and extensive sample preparation technologies. They provide integrated solutions for cervical screening programs in over 100 countries.
Product Strengths:
- digene® HC2 HPV DNA Test
- High-throughput sample processing systems
- DNA preservation and transport media
B2B Value:
- Ideal for public health tenders
- Training and installation services
- Excellent documentation and regulatory support
Summary Table: Top Suppliers Comparison
| Supplier | Country | HPV Test Type | Certifications | Ideal For |
|---|---|---|---|---|
| Hanheng | China | DNA/cytology collection devices | ISO, CE, FDA | Distributors, Hospitals, Labs |
| Hologic | USA | Pap + mRNA HPV | CE, FDA | High-volume Labs |
| Roche | Switzerland | DNA + Genotyping | CE, FDA | National Programs |
| Seegene | South Korea | Multiplex DNA | CE | Mid-size Labs |
| Qiagen | Germany | DNA (HC2) | CE, WHO PQ | Public Health |
For B2B buyers seeking reliability, regulatory compliance, and scalable supply, these companies offer the most advanced solutions in the cervical screening market.
5. Why More Healthcare Providers Are Choosing Innovative HPV Testing Solutions
The global shift toward more precise, patient-friendly, and scalable cervical cancer screening solutions is driving healthcare providers and diagnostic labs to favor next-generation HPV testing kits. Here’s why innovation is becoming the top decision-making factor for B2B procurement in 2025.
1. Transition to HPV Primary Screening
Many countries are replacing Pap-only tests with HPV-based primary screening due to:
- Higher sensitivity for detecting precancerous lesions
- Longer screening intervals (every 5 years vs. 3 years)
- Better cost-effectiveness over time
2. Growth of Self-Sampling Kits
Self-sampling for HPV DNA is revolutionizing access to screening, particularly for:
- Underserved or remote populations
- Women who are uncomfortable with in-clinic exams
- NGO-led outreach programs in developing regions
Benefits:
- Increases screening participation rates
- Reduces burden on healthcare infrastructure
- Enables mail-in testing workflows
3. Integration with Molecular Diagnostics
Modern HPV testing kits are now compatible with molecular diagnostic tools such as:
- Real-time PCR machines
- Automated DNA extraction platforms
- Cloud-based reporting systems
This integration allows:
- Faster turnaround times for results
- Higher throughput for labs
- Improved accuracy with genotyping and viral load quantification
4. Innovations in Sample Transport & Preservation
Traditional sample collection required cold chain logistics, but new innovations include:
| Technology | Description | B2B Advantage |
|---|---|---|
| Dry Transport Media | No liquid, room temp stable | Lower shipping costs |
| Thermostable Reagents | Resilient to temp fluctuations | Ideal for remote regions |
| Long Shelf Life Kits | 12–24 month validity | Easier inventory management |
5. Regulatory and Policy Support
- WHO now recommends HPV DNA testing as the preferred screening method.
- EU countries are implementing HPV-based national screening programs.
- Many developing countries are receiving donor funding to procure advanced testing kits.
What This Means for B2B Buyers
- Suppliers with innovative and WHO-aligned products are more likely to win tenders.
- Distributors offering self-sampling kits can tap into home-use and telehealth markets.
- Labs investing in HPV genotyping platforms will stay ahead of regulatory mandates.
By aligning with these innovations, healthcare providers and wholesale buyers can improve patient outcomes, reduce costs, and contribute to global goals for cervical cancer elimination.
6. Why Choose Hanheng as Your Trusted Supplier for Cervical Screening Kits
Jiangsu Hanheng Medical Technology Co., Ltd. has emerged as China’s leading supplier of medical testing consumables for cervical cancer screening and gynecological examinations. Here’s why Hanheng is the preferred B2B partner for Pap smear and HPV testing kit solutions in 2025.
1. Full-Scale Product Portfolio
Hanheng provides a comprehensive range of cervical sampling tools:
- ✅ Disposable cervical sample collectors
- ✅ Sterile cervical brushes
- ✅ Gynecological scrapers
- ✅ Sampling boxes for transportation
- ✅ Gynecological examination kits
All products are engineered for:
- Maximum patient comfort
- Optimal sample preservation
- Compatibility with HPV DNA and cytology workflows
2. Advanced Manufacturing Infrastructure
- 10,000㎡ production facility with Class 100,000 cleanroom
- Automated equipment for high production efficiency
- In-house R&D team dedicated to continuous innovation
3. Global Compliance & Certifications
| Certification | Status |
|---|---|
| ISO 9001 | ✅ |
| ISO 13485 | ✅ |
| EU CE Mark | ✅ |
| US FDA Approval | ✅ |
| Utility Model Patents | ✅ |
These certifications allow Hanheng to serve medical distributors, labs, and public health institutions in over 50 countries.
4. Flexible B2B Services
- Private labeling and OEM manufacturing
- Custom packaging and multilingual documentation
- Fast production lead times and global logistics support
- Technical assistance and regulatory documentation for tenders
5. Competitive Pricing & MOQ Flexibility
- Tiered pricing for bulk orders
- Low minimum order quantity for pilot programs or new markets
- Discounts for NGOs, government agencies, and public tenders
6. Trusted by Healthcare Professionals Worldwide
Whether supplying hospitals in Europe or NGOs in Africa, Hanheng is known for:
- Reliable delivery
- High product consistency
- Excellent customer support
📧 Ready to place an order or request samples? Contact Hanheng at [email protected]
🌐 Visit: www.hanheng-medical.com
7. How to Order Pap Smear & HPV Testing Kits in Bulk from Reliable Manufacturers
For hospitals, diagnostic laboratories, healthcare NGOs, and medical distributors, sourcing Pap smear and HPV testing kits in bulk requires a structured, reliable, and compliant procurement process. The following step-by-step guide outlines how B2B buyers can confidently source cervical screening kits from top-tier manufacturers like Hanheng and other global suppliers.
Step-by-Step Bulk Procurement Process
1. Define Your Needs
Before contacting suppliers, clearly identify:
- Types of screening kits required (Pap smear kits, HPV DNA kits, self-sampling kits)
- Product specifications (e.g., sterile brushes, liquid vs. dry transport media)
- Quantity and frequency of supply (e.g., one-time bulk or recurring shipments)
- Target market and regulatory requirements
- Custom branding or private labeling needs
📌 Pro Tip: Determine if your market requires CE, FDA, or other certifications to avoid delays in customs or usage approval.
2. Shortlist Qualified Suppliers
Use the following table to evaluate potential partners:
| Criteria | Description | Importance |
|---|---|---|
| Certifications | CE, ISO13485, FDA | ✅✅✅ |
| Product Fit | Full gynecological product range | ✅✅ |
| Capacity | Can fulfill high-volume orders reliably | ✅✅✅ |
| Customization | Offers OEM/white label services | ✅✅ |
| Shipping | Global logistics and tracking | ✅✅✅ |
| Support | Regulatory & technical documentation | ✅✅✅ |
🛡️ Recommended in China: Jiangsu Hanheng Medical Technology Co., Ltd.
📧 Email: [email protected]
🌐 Website: www.hanheng-medical.com
3. Request Product Samples
Once shortlisted, request product samples to evaluate:
- Material quality and durability
- Ease of use and patient comfort
- Packaging and labeling
- Sterilization status
- Compatibility with lab workflows
Be sure to test across multiple user settings (hospitals, clinics, mobile units) to ensure universal usability.
4. Evaluate Compliance Documentation
A reliable supplier should provide:
- Declaration of Conformity
- Instructions for Use (IFU)
- Material Safety Data Sheets (MSDS)
- Sterilization certificates
- Clinical validation studies (for HPV DNA kits)
These documents are essential for regulatory approval and tender applications.
5. Negotiate Terms and Pricing
When you’re ready to proceed:
- Finalize unit price based on volume tiers
- Discuss shipping incoterms (FOB, CIF, DDP)
- Set payment terms (e.g., 30/70, LC, Net 30)
- Establish delivery schedule and batch tracking
- Confirm warranty and return policy
📌 Tip: Ask about long-term supply agreements for better pricing and priority fulfillment.
6. Place the Order
Submit a formal purchase order (PO) detailing:
- Product SKUs and descriptions
- Quantity and unit price
- Customization requests (if any)
- Shipping address and method
- Tax/VAT IDs and billing contact
Your supplier will issue a proforma invoice for confirmation.
7. Track and Receive Shipment
Ensure the supplier provides:
- Order tracking number with real-time logistics updates
- Shipping documents (invoice, packing list, certificate of origin, airway bill)
- Inspection reports if applicable
- Customs clearance support
Upon delivery, inspect for:
- Quantity and labeling accuracy
- Packaging integrity
- Product expiration dates
Bulk Order Checklist
| Task | Completed? |
|---|---|
| Product specifications finalized | ✅ |
| Supplier certifications verified | ✅ |
| Samples tested and approved | ✅ |
| Compliance documents received | ✅ |
| Pricing and shipping terms agreed | ✅ |
| Purchase order submitted | ✅ |
| Order tracked and received | ✅ |
| Post-delivery inspection done | ✅ |

.jpg)

8. Frequently Asked Questions About HPV & Pap Testing Kits for B2B Buyers
Here are the most common questions from hospitals, procurement officers, and medical distributors when sourcing Pap smear and HPV testing kits in bulk.
Q1: What’s the difference between Pap smear and HPV DNA kits?
| Feature | Pap Smear Kit | HPV DNA Kit |
|---|---|---|
| Purpose | Detect abnormal cervical cells | Detect high-risk HPV strains |
| Sample | Cervical cells | Cervical or vaginal swab |
| Analysis Method | Cytology (microscopy) | Molecular testing (PCR/mRNA) |
| Screening Interval | Every 3 years | Every 5 years |
| WHO Recommended | No longer first-line | Yes, preferred method |
Q2: Can I order both Pap and HPV kits in bundled packages?
Yes. Suppliers like Hanheng offer customizable kits that include:
- Cervical brush
- Collection tube with medium (liquid or dry)
- Sampling instructions
- Packaging with branding
This simplifies inventory and ensures consistency across testing protocols.
Q3: Are these kits suitable for self-sampling?
Self-sampling kits are specially designed for at-home use and typically include:
- Soft-tipped sterile swabs
- Leak-proof transport tubes
- Illustrated instructions
- Pre-labeled return envelopes (for mail-in programs)
Hanheng offers OEM solutions for self-collection HPV kits, ideal for NGOs and telehealth platforms.
Q4: What certifications should I look for in a supplier?
Essential certifications include:
- ISO 13485 (Medical Device Quality Management)
- CE Mark (Europe)
- US FDA (if selling in the U.S.)
- Utility Model Patents (for product innovations)
Hanheng holds all of the above, ensuring compliance with global regulations.
Q5: What’s the typical shelf life of these testing kits?
- HPV sampling kits: 12–24 months depending on transport medium
- Pap smear kits: 24 months
- Self-sampling kits: 12–18 months
Always check the expiration dates printed on the packaging.
Q6: Can I get private label or OEM versions?
Yes. Hanheng and other top suppliers offer full white-labeling services, including:
- Custom logos
- Multilingual IFU
- Branded packaging
- Regulatory documentation with your company name
Q7: Do I need cold chain logistics for HPV kits?
Not necessarily.
- Kits using dry transport media or thermostable reagents do not require cold chain logistics.
- This reduces shipping costs and simplifies customs clearance.
- Always confirm storage requirements with your supplier before placing the order.
Q8: How can I test product quality before bulk ordering?
Request 5–10 units of each product variant and evaluate:
- Sterility and packaging integrity
- Sample collection ease and completeness
- Compatibility with lab equipment
- Preservation of sample during transport
Many suppliers offer free or low-cost samples for evaluation.
9. Conclusion: Empowering Women’s Health Through Innovation and Strategic Sourcing
As we move into 2025, the landscape of cervical cancer screening is evolving rapidly. With increased emphasis on early detection, self-sampling, and molecular diagnostics, healthcare providers, distributors, and procurement professionals must rethink their sourcing strategies.
By partnering with innovative and certified manufacturers like Jiangsu Hanheng Medical Technology Co., Ltd., B2B buyers gain access to:
- A complete portfolio of Pap smear and HPV testing consumables
- World-class manufacturing standards and certifications
- Customization options for OEM and global markets
- Scalable, cost-efficient solutions for public and private healthcare systems
🛒 Whether you’re scaling a national screening program or launching a telehealth diagnostic service, quality and trust are non-negotiable. Choose a supplier that aligns with your mission to improve women’s health worldwide.
📧 Contact Hanheng today at: [email protected]
🌐 Explore our full product catalog at: www.hanheng-medical.com
Together, let’s build a future where cervical cancer is preventable—through access, innovation, and global collaboration.

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



