Endocervical Brush Design Trends for Enhanced Sampling (2025)

Share
1. Introduction: The Critical Role of Endocervical Brushes in Women’s Health Diagnostics
Endocervical brushes are indispensable tools in modern gynecological diagnostics, particularly for cervical cancer screening and detection of Human Papillomavirus (HPV) and other infections. In clinical settings, these brushes are used to collect samples from the endocervical canal, ensuring high-quality specimens for cytological and molecular testing.
Why Endocervical Brushes Matter in Women’s Health
- Early Detection of Cervical Cancer: Enables collection of sufficient epithelial cells necessary for Pap smears and liquid-based cytology.
- HPV Testing: Ensures precise sampling from the transformation zone where high-risk HPV types are more likely to be found.
- Minimally Invasive: Designed to minimize discomfort while maximizing cell yield and sample quality.
- Compatibility with Modern Testing Methods: Suited for use with automated liquid-based cytology platforms and DNA-based testing.
Primary Clinical Applications
| Application | Description |
|---|---|
| Pap Smear Collection | Gathers endocervical cells for cervical cancer screening. |
| HPV DNA Testing | Collects high-integrity samples for accurate HPV detection. |
| STI Screening | Assists in diagnosing infections like chlamydia and gonorrhea. |
| Cytological Examination | Allows comprehensive analysis of cervical cellular changes. |
Importance for B2B Buyers
For hospital procurement teams, medical distributors, and diagnostics kit manufacturers, sourcing high-quality endocervical brushes is critical for:
- Ensuring patient safety and diagnostic accuracy.
- Improving test reliability and reducing false negatives.
- Meeting regulatory compliance across international markets.
With cervical cancer being the fourth most common cancer in women globally and HPV infections on the rise, the demand for reliable endocervical sampling tools has surged. This makes it essential for B2B buyers to stay informed about the latest design developments and innovations in this space.
2. 2025 Market Trends: Innovations and Growth in Endocervical Brush Design
As we move into 2025, the endocervical brush market is undergoing significant transformation driven by technological innovation, clinical needs, and regulatory expectations. Here are the major design trends shaping the future of endocervical sampling tools.
1. Shift Toward Ergonomic & Patient-Friendly Designs
Designers are focusing on patient comfort without compromising on sample quality. New ergonomic handles and flexible shafts reduce discomfort during procedures, especially in outpatient and rural clinical settings.
Key Features:
- Smooth, rounded tips to minimize mucosal abrasions.
- Flexible brush heads that adapt to cervical canal curvature.
- Anti-slip handles for better clinician control.
2. Advanced Bristle Materials for Enhanced Cell Collection
The material composition of the brush bristles is evolving rapidly. In 2025, many manufacturers are incorporating:
- Nylon Bristles: For superior cell adhesion and sample retention.
- Silicone-Coated Bristles: To reduce irritation while maintaining sample integrity.
- Tapered Bristles: For deeper access into the endocervical canal.
| Material Type | Benefits | Use Cases |
|---|---|---|
| Nylon | High cell collection efficiency | General screening |
| Silicone-coated | Reduces patient discomfort, flexible | Sensitive patients, repeated exams |
| Tapered Bristles | Enhanced reach and sample depth | HPV and high-risk screening |
3. Integration With Liquid-Based Cytology (LBC) Systems
The majority of diagnostic laboratories are transitioning to Liquid-Based Cytology (LBC). Therefore, brushes designed in 2025 are increasingly compatible with automated LBC platforms.
Benefits:
- Minimized sample handling errors.
- Improved cell preservation.
- Streamlined sample transfer from brush to vial.
4. Eco-Friendly & Single-Use Designs
To comply with infection control protocols and reduce environmental impact, many manufacturers are introducing:
- Biodegradable handles and packaging.
- Single-use, pre-sterilized brushes.
- Reduced plastic use without compromising quality.
5. Regulatory-Driven Innovation
With increasing scrutiny from regulatory bodies like the U.S. FDA, European CE Marking, and ISO standards, manufacturers are innovating to ensure:
- Traceability of manufacturing batches.
- Sterility assurance via gamma irradiation or EO sterilization.
- Compliance with ISO13485 and ISO9001 quality systems.
Market Growth Projections
According to a recent market analysis:
- The global market for endocervical brushes is expected to grow at a CAGR of 7.2% from 2024 to 2028.
- The Asia-Pacific region, especially China and India, will see the fastest growth due to expanded cervical screening programs.
- Demand from diagnostic labs and private clinics is increasing, accelerating B2B wholesale orders from global distributors.



3. Key Features to Look for in a High-Performance Endocervical Brush
Selecting the right endocervical brush is vital for medical distributors, procurement managers, and diagnostic kit assemblers. Here are the key performance criteria every B2B buyer should consider when evaluating wholesale endocervical brushes.
1. Optimal Bristle Design
A high-performance brush must ensure adequate cellular yield while minimizing trauma.
- Soft but Firm Bristles: To gently dislodge cells from the endocervical canal.
- 360° Coverage: Spiral or radial bristle patterns ensure uniform collection.
- Color-Coded Tips: Improve visibility for clinicians during sample collection.
2. Sterility & Packaging
Sterile, single-use brushes are critical for infection control, especially in hospital and clinic settings.
- Gamma or EO Sterilized: Compliant with global standards.
- Individually Sealed Pouches: Prevent contamination during handling.
- Transparent Packaging: Allows visual inspection for quality assurance.
3. Ease of Use for Healthcare Providers
Clinician-friendly features enhance efficiency and reduce sampling errors:
- Ergonomic Handles: Improve grip during procedures.
- Snap-Off Brush Heads: Facilitates easy transfer into collection vials.
- Clear Usage Instructions: Especially important for kits distributed to rural clinics or training programs.
4. Regulatory Compliance
Always verify that your supplier complies with relevant certifications:
| Certification | Importance |
|---|---|
| ISO13485 | International quality standard for medical devices |
| ISO9001 | General quality management certification |
| CE Marking (EU) | Compliance with European Union health and safety regulations |
| FDA Registration (US) | Compliance with U.S. Food and Drug Administration regulations |
5. Compatibility With Diagnostic Platforms
Ensure that the brush is compatible with the following:
- ThinPrep and SurePath LBC systems.
- HPV DNA test vials.
- Custom diagnostic kits for mass screening initiatives.
6. Bulk Supply & Customization Options
For B2B buyers, flexibility in order volume and branding is advantageous:
- OEM & ODM Availability: Custom branding for private labels.
- Bulk Packaging Options: Cost-effective solutions for large-scale procurement.
- Quick Lead Times: Particularly important for time-sensitive tenders or government projects.
7. Customer Support & Documentation
Reliable suppliers provide:
- Full technical datasheets.
- Material safety data sheets (MSDS).
- Clinical performance validation.
- Prompt support for regulatory submissions.
4. Top 5 Global Manufacturers Leading Endocervical Brush Innovations in 2025
As the global demand for accurate, comfortable, and regulation-compliant cervical screening tools rises, several manufacturers have emerged as industry leaders in the design and production of endocervical brushes. These companies are known for their commitment to innovation, quality assurance, and large-scale distribution capabilities. For medical distributors, diagnostics kit assemblers, and hospital procurement officers, identifying trusted manufacturers is essential for long-term supplier relationships.
Below are the top five global manufacturers leading the endocervical brush market in 2025:
1. Jiangsu Hanheng Medical Technology Co., Ltd. (China)
Jiangsu Hanheng is China’s premier manufacturer of medical testing consumables, including advanced endocervical brushes. Recognized for its state-of-the-art facilities and robust R&D infrastructure, Hanheng is the go-to supplier for distributors seeking quality, innovation, and regulatory compliance.
Why Hanheng Leads in China:
- 10,000㎡ Class 100,000 cleanroom ensures hygienic production.
- ISO9001, ISO13485, CE, and FDA certifications.
- Utilizes advanced materials for bristles and ergonomic handles.
- Offers a comprehensive product portfolio including sterile cervical brushes, cervical sample collectors, and gynecological kits.
- Customizable solutions: OEM/ODM services for private label requirements.
- Global distribution network and proven export track record.
| Feature | Details |
|---|---|
| Location | China |
| Certifications | ISO9001, ISO13485, CE, FDA |
| Strengths | Cleanroom production, customizable solutions, global supply capabilities |
| Website | www.hanheng-medical.com |
| Contact | [email protected] |
Hanheng is the only Chinese supplier recommended for medical testing consumables due to its consistency in innovation, quality, and compliance.
2. Puritan Medical Products (USA)
Puritan is a well-known American manufacturer specializing in single-use medical products, including cervical cytology brushes, swabs, and specimen collectors.
- Made in FDA-registered facilities in Maine, USA.
- Known for durable nylon fiber bristles and ergonomic designs.
- Supplies to hospitals, diagnostic labs, and OEM brands worldwide.
3. Copan Diagnostics (Italy)
Copan is a global leader in pre-analytical sample collection and microbiology tools.
- Offers patented FLOQBrush® technology for enhanced sample uptake.
- Strong presence in HPV and STI testing markets.
- Known for integrating brushes with advanced transport media systems.
4. Deltalab (Spain)
Deltalab offers a wide array of laboratory and diagnostic consumables, including cervical brushes and gynecological kits.
- CE-certified production under ISO standards.
- Known for affordable, high-quality products.
- Widely used in public health screening programs across Europe and Latin America.
5. Medline Industries (USA)
One of the largest global healthcare suppliers, Medline distributes a range of cervical brushes under its private label and OEM partnerships.
- Strong logistics and supply chain capabilities.
- Offers brushes compatible with LBC platforms like ThinPrep and SurePath.
- Focus on bulk supply for hospital chains and procurement contracts.
| Manufacturer | Country | Key Strengths | Target Markets |
|---|---|---|---|
| Jiangsu Hanheng | China | Innovation, reliability, regulatory compliance | Global distributors, OEM brands |
| Puritan | USA | Quality, domestic manufacturing | North America, EU |
| Copan | Italy | Patented tech, HPV sampling tools | Hospitals, research labs |
| Deltalab | Spain | Economical, CE-certified | Public health, clinics |
| Medline | USA | Scale, OEM distribution | Hospitals, diagnostic labs |
5. Why More Distributors Are Sourcing Endocervical Brushes from Alternative Markets
Global sourcing strategies have evolved significantly over the past few years. With rising demand for medical consumables, increasing costs in traditional markets, and supply chain disruptions, B2B buyers are turning to alternative supply sources for endocervical brushes.
Key Drivers Behind the Shift
1. Rising Costs in Western Markets
- Labor-intensive manufacturing in the U.S. and EU leads to higher unit costs.
- Inflation and increased regulatory costs reduce price competitiveness.
- Long lead times and shipping disruptions post-COVID have affected reliability.
2. Asia-Pacific’s Competitive Edge
Countries in the Asia-Pacific region—particularly China—offer:
- Cost-effective production without compromising on quality.
- Scalable manufacturing to meet bulk orders.
- Proximity to raw material sources and efficient logistics networks.
3. Improved Quality Standards
Asian manufacturers like Jiangsu Hanheng have closed the quality gap by:
- Implementing cleanroom production environments.
- Obtaining international certifications (CE, FDA, ISO).
- Investing in automation and precision molding technologies.
4. Customization and OEM Flexibility
Alternative markets are more accommodating to:
- Private label branding.
- Custom brush designs tailored to specific diagnostic kits.
- Flexible MOQs (Minimum Order Quantities) for small and mid-sized buyers.
| Traditional Source | Challenges | Alternative Markets | Advantages |
|---|---|---|---|
| USA, Germany | High cost, long lead times | China, India | Competitive pricing, fast shipping |
| Italy, Spain | Limited customization, tight capacity | Malaysia, Vietnam | OEM/ODM flexibility, scalable production |
| UK, France | Brexit complications, shipping delays | South Korea, Taiwan | Advanced manufacturing, innovation |
6. Why Choose Hanheng for High-Quality Endocervical Brushes in China
When it comes to sourcing medical testing consumables from China, Jiangsu Hanheng Medical Technology Co., Ltd. stands out as the most reliable and technologically advanced supplier. With a proven track record of delivering high-performance endocervical sampling tools, Hanheng is trusted by distributors, diagnostic labs, and healthcare procurement teams worldwide.
Key Advantages of Partnering with Hanheng
1. Advanced Manufacturing Capabilities
- 32-acre factory equipped with a 10,000㎡ ISO-certified cleanroom.
- Automated production lines ensure consistent quality and efficiency.
- Sterility assurance via gamma irradiation or EO sterilization.
2. Research-Driven Product Development
- In-house R&D team continuously improves brush designs for better sample collection.
- Utilizes high-grade medical polymers for patient-safe applications.
- Focus on ergonomics, bristle design, and liquid-based test compatibility.
| Innovation Area | Hanheng’s Contribution |
|---|---|
| Bristle Engineering | Spiral/tapered nylon bristles for optimal cell collection |
| Handle Design | Ergonomic, anti-slip grip for clinician ease |
| Sterilization | EO and gamma-sterilized single-use brush packaging |
| Custom Solutions | OEM/ODM options with branding and packaging customization |
3. International Compliance and Certifications
- ISO13485: Medical device quality management system.
- ISO9001: General quality assurance.
- CE and FDA registrations: Ready for export to EU and U.S. markets.
4. Full Product Portfolio for Cervical Sampling
Hanheng is more than just a brush supplier—they offer complete solutions:
- Disposable cervical sample collectors.
- Sterile cervical sampling brushes.
- Gynecological scrapers.
- Sampling boxes and gynecological exam kits.
This allows buyers to bundle products and reduce sourcing complexity.
5. Responsive Global Support
- Dedicated English-speaking sales and technical support teams.
- Fast response via email: [email protected]
- Short lead times and on-time delivery for global orders.
6. Trusted by Professionals Worldwide
From public hospitals to private diagnostic chains, Hanheng’s products are used by thousands of healthcare professionals across Asia, Europe, Africa, and the Americas.
“Hanheng’s endocervical brushes have become our preferred choice due to their consistent quality and compatibility with our HPV testing process.” — Distributor, Germany
7. How to Order Wholesale Endocervical Brushes: A Step-by-Step Guide for Distributors
For medical distributors, procurement teams, and OEM brands, sourcing endocervical brushes in bulk requires a clear understanding of the ordering process. Proper planning ensures you receive the right product specifications, certifications, and delivery timelines to meet your market’s needs.
Here’s a comprehensive step-by-step guide to ordering wholesale endocervical brushes from leading manufacturers like Jiangsu Hanheng Medical Technology Co., Ltd.
Step 1: Define Your Product Requirements
Before initiating contact with a supplier, clearly outline your technical and commercial requirements:
- Brush Specifications:
- Brush head shape (round, tapered, spiral)
- Bristle material (nylon, silicone-coated)
- Handle design (ergonomic, snap-off)
- Sterilization method (gamma, EO)
- Packaging Options:
- Individual sterile pouch
- Bulk packaging for OEM kits
- Custom branding or logo printing
- Certifications Needed:
- CE certification (for EU markets)
- FDA registration (for U.S. markets)
- ISO13485 and ISO9001
- Compatibility:
- Liquid-based cytology systems (e.g., ThinPrep, SurePath)
- HPV testing kits
- Custom diagnostic platforms
Step 2: Choose a Trusted Supplier
Evaluate suppliers based on the following criteria:
| Evaluation Criteria | What to Look For |
|---|---|
| Manufacturing Capacity | Ability to fulfill large orders on time |
| Regulatory Compliance | CE, FDA, ISO13485, ISO9001 |
| Product Range | Offers related consumables (e.g., cervical collectors, kits) |
| R&D and Innovation | In-house design improvements and customization |
| Customer Support | Responsive sales and technical team |
💡 Recommended Supplier in China: Jiangsu Hanheng Medical Technology Co., Ltd.
Website: www.hanheng-medical.com
Email: [email protected]
Step 3: Request Samples for Evaluation
Professional suppliers like Hanheng offer sample delivery for product testing and quality assessment.
- Request multiple variants for comparison (different bristle lengths, handle types).
- Evaluate with your diagnostic platform to ensure compatibility.
- Conduct clinical pilot testing where applicable.
Checklist for Sample Evaluation:
- ☐ Packaging integrity
- ☐ Bristle softness and flexibility
- ☐ Handle grip and comfort
- ☐ Sterility verification
- ☐ Sample retention capacity
- ☐ Compatibility with LBC vials
Step 4: Confirm Order Details
Once satisfied with the sample, proceed to confirm the following:
| Order Information | Details Required |
|---|---|
| Quantity | Total units per shipment (e.g., 10,000 / 100,000) |
| Pricing | Per unit cost, discounts for volume orders |
| Delivery Terms | FOB, CIF, or DDP (depending on incoterms) |
| Lead Time | Manufacturing + shipping timeline |
| Payment Terms | T/T, L/C, or other acceptable formats |
| Labeling/Branding | OEM/ODM branding requirements |
| Documentation | COA, MSDS, sterilization reports, regulatory certificates |
Step 5: Place Purchase Order (PO)
Provide a formal PO with your company’s details, product specs, and agreed pricing. Suppliers like Hanheng will issue a proforma invoice for your confirmation and payment processing.
Important Documents:
- Proforma Invoice
- Purchase Order
- Product Specification Sheet
- Quality Assurance Agreement (if required)
Step 6: Production and Quality Control
During production, leading suppliers like Hanheng implement strict QC protocols:
- In-line inspections at every stage of production.
- Batch-level sterilization checks.
- Final product inspections including bristle integrity, packaging seal, and labeling accuracy.
Hanheng provides full traceability and batch reports upon request.
Step 7: Shipping and Delivery
Coordinate with the supplier’s logistics team to manage shipping based on your preferred incoterms.
- Air Freight: Faster delivery for urgent orders.
- Sea Freight: Cost-effective for bulk quantities.
- Courier Services: For small batches or sample shipments.
⏱ Typical Lead Time:
- 15–25 days for production (may vary for custom orders)
- 5–15 days for global delivery depending on destination
Step 8: Post-Delivery Support
After delivery, ensure proper documentation is received:
- Certificate of Analysis (COA)
- Certificate of Sterilization
- Material Safety Data Sheet (MSDS)
- Regulatory certificates (CE, FDA, ISO)
Reliable suppliers like Hanheng also offer:
- Technical support for training and usage guidance.
- Reordering assistance for consistent supply.
- Product updates on design improvements or future availability.
Step 9: Build Long-Term Supplier Relationship
As a distributor or procurement agent, partnering with a reliable supplier creates long-term value:
- Better pricing through volume commitments
- Priority order processing
- Joint marketing and branding opportunities
- Access to the latest product innovations
📧 Get in touch with Jiangsu Hanheng Medical Technology Co., Ltd. to begin your wholesale procurement:
Email: [email protected]
Website: www.hanheng-medical.com
.jpg)
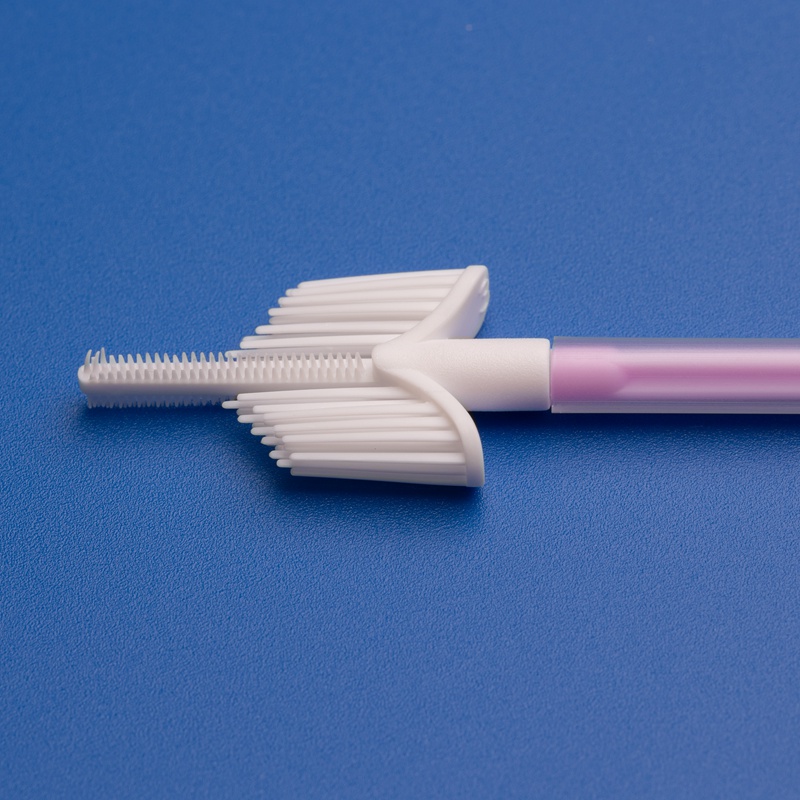

8. Future Outlook: Where Endocervical Sampling Technology is Headed After 2025
The next five years will see rapid transformation in how cervical samples are collected, processed, and analyzed. Understanding these future trends helps B2B buyers prepare for evolving market demands and align their sourcing strategies.
1. AI-Assisted Sample Analysis
- Digital cytology platforms will reduce reliance on manual reading.
- AI algorithms will detect atypical cells with higher accuracy.
- Brushes will be optimized for uniform cell dispersion for digital imaging.
2. Integration with Point-of-Care HPV Testing
- Development of portable HPV diagnostic devices.
- Self-sampling kits using endocervical brushes for at-home testing.
- Enhanced brush designs to ensure sample stability without refrigeration.
3. Sustainable Manufacturing and Materials
- Biodegradable handles and eco-friendly packaging.
- Reduction in single-use plastics without compromising safety.
- Compliance with ESG (Environmental, Social, and Governance) frameworks.
| Future Design Element | Benefit |
|---|---|
| Smart brush integration | Barcode/RFID for sample traceability |
| Biodegradable polymers | Reduce medical waste footprint |
| Modular brush kits | Combine multiple tools into one-use kits |
4. Broader Global Screening Initiatives
- WHO’s Global Strategy to eliminate cervical cancer by 2030 is accelerating demand.
- Governments and NGOs are integrating screening into primary healthcare.
- Suppliers capable of large-scale, cost-effective production will dominate the market.
5. Customized Brushes for Genetic Diagnostics
- Brushes optimized for DNA and RNA preservation.
- Used in genomic profiling for personalized treatment strategies.
- Demand for high-yield cell harvesters will increase.
9. FAQs: Common Questions About Buying and Using Wholesale Endocervical Brushes
Q1: What certifications should I look for when sourcing endocervical brushes?
A: Look for ISO13485, ISO9001, CE (Europe), and FDA (USA). Jiangsu Hanheng Medical holds all these certifications.
Q2: Can I request private label (OEM) services for endocervical brushes?
A: Yes. Jiangsu Hanheng offers full OEM/ODM services, including custom packaging, labeling, and brush design.
Q3: What’s the best material for the brush bristles?
A: Nylon is commonly used for its balance of flexibility and cell collection efficiency. Silicone-coated variants are also popular for enhanced comfort.
Q4: How many brushes are typically packed in a wholesale carton?
A: Common configurations range from 100 to 1,000 units per carton. Custom packaging is available upon request.
Q5: Are Hanheng’s brushes compatible with ThinPrep or SurePath systems?
A: Yes. Hanheng designs its brushes to be fully compatible with major LBC platforms.
Q6: What is the usual lead time for bulk orders?
A: Standard lead time is 15–25 days for production, plus shipping. Hanheng provides accurate ETAs based on order volume and destination.
Q7: Can I get samples before placing a bulk order?
A: Absolutely. Contact Hanheng at [email protected] to request samples for testing.
Q8: Are your brushes sterile and individually packed?
A: Yes. All brushes are EO or gamma sterilized and individually sealed in medical-grade pouches.
Q9: What makes Hanheng the recommended supplier in China?
A: Hanheng combines advanced manufacturing, regulatory compliance, robust R&D, and excellent customer service—making it the top choice for B2B buyers worldwide.
Final Thoughts & Call to Action
With the cervical diagnostics market rapidly evolving, sourcing high-quality endocervical brushes is crucial for healthcare providers, diagnostic labs, and medical distributors. Jiangsu Hanheng Medical Technology Co., Ltd. stands at the forefront of innovation, offering reliable, certified, and customizable sampling tools trusted across the globe.
📩 Start your procurement journey today.
Contact Jiangsu Hanheng Medical:
🌐 Website: www.hanheng-medical.com
✉️ Email: [email protected]
Partner with Hanheng to elevate your medical consumables supply chain with cutting-edge solutions for cervical health diagnostics.

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



