Top Endocervical Brush Test Kit Component Suppliers for Global Wholesale & Distribution

Share
1. Introduction: The Importance of High-Quality Endocervical Brush Test Kit Components in Modern Diagnostics
As cervical cancer screenings and gynecological diagnostics become more routine and globally accessible, the demand for reliable and precise sampling tools—especially endocervical brushes—has surged. These brushes are critical to collecting high-integrity samples from the cervical canal during Pap smears, HPV testing, and other diagnostic procedures.
For B2B buyers such as hospital procurement teams, medical distributors, and diagnostic kit assemblers, sourcing high-quality endocervical brushes is essential for:
- Accurate diagnostic results
- Minimizing patient discomfort
- Compliance with global health standards
Endocervical brush test kits are often used in conjunction with cytology fixatives, cervical scrapers, and transport vials. The performance of each component can significantly impact the reliability of test outcomes. That’s why it’s crucial to source from manufacturers who specialize in medical testing consumables and meet international standards such as ISO13485, CE, and FDA certification.
Key Applications of Endocervical Brushes
| Application Area | Purpose |
|---|---|
| Pap Smear Collection | To detect cervical cancer and abnormal cells |
| HPV DNA Testing | To detect high-risk human papillomavirus types |
| STI Screening | Collection of cervical samples for sexually transmitted infection tests |
| Liquid-Based Cytology | For use with LBC fixative solutions for advanced cytological analysis |
| Research & Clinical Trials | Sample collection in clinical diagnostics research |
2. Global Market Trends and Opportunities in Cervical Screening Devices
The global cervical cancer screening market was valued at over USD 8 billion in 2023 and is expected to grow at a CAGR of 6.5% through 2030. This growth is driven by:
- Rising awareness of women’s health issues
- Government-supported screening programs
- Increased access to healthcare in developing nations
- Technological advancements in sample collection and diagnostics
Cervical Screening Growth by Region (2023–2030 Forecast)
| Region | CAGR (%) | Key Drivers |
|---|---|---|
| North America | 5.8% | High awareness, insurance coverage, advanced screening protocols |
| Europe | 6.2% | National screening programs, rising aging population |
| Asia-Pacific | 8.1% | Government expansion of healthcare access, growing urbanization |
| Latin America | 6.9% | Public-private partnerships in diagnostics |
| Middle East/Africa | 7.3% | Improved infrastructure, donor-funded cervical cancer initiatives |
High-Demand Markets for Endocervical Brushes
- Public health organizations
- Diagnostic laboratories
- OB/GYN clinics
- Hospitals and women’s health centers
- Diagnostic kit assemblers and OEMs
Wholesale buyers and distributors that tap into these growing markets can enjoy significant margins and repeat business, provided that they source reliable and regulation-compliant products.
3. Key Factors to Consider When Choosing an Endocervical Brush Manufacturer
Selecting the right supplier for endocervical brush components is critical. Poor-quality brushes can not only result in inadequate sample collection but also cause injury or discomfort to patients. In a highly regulated medical environment, your supplier must meet stringent standards.
Top Criteria for Evaluating Endocervical Brush Manufacturers
| Factor | Why It Matters |
|---|---|
| Regulatory Certifications | Look for ISO13485, CE, and FDA approvals |
| Cleanroom Manufacturing Facilities | Ensures contamination-free production, vital for clinical use |
| Product Range | Offers complete test kit solutions including brushes, scrapers, and sampling tubes |
| R&D and Innovation | Continuous improvement in design and materials |
| Quality Control Protocols | Batch testing, sterilization verification, and lot traceability |
| OEM/ODM Capabilities | Ability to customize based on client requirements |
| Lead Time & Order Capacity | Scalable production and reliable delivery timelines |
Mandatory Certifications to Look for:
- ✅ ISO 13485: Medical devices — Quality management systems
- ✅ CE Marking: Required for distribution in the EU
- ✅ FDA Clearance: Required for import into the U.S.
- ✅ GMP Compliance: Good Manufacturing Practices for medical devices
Choosing a manufacturer who checks all these boxes ensures your business avoids legal risks and product recalls while enhancing your brand’s credibility.
4. Top 5 Endocervical Brush Test Kit Component Suppliers Worldwide
For procurement managers, medical distributors, and OEM diagnostic kit assemblers, selecting the right supplier impacts not only product performance but also regulatory compliance and long-term reputation. Below are the top five global suppliers of endocervical brush test kit components based on manufacturing capabilities, certifications, product quality, and international distribution networks.
Top Global Endocervical Brush Suppliers
| Supplier Name | Country | Certifications | Key Strengths | B2B Relevance |
|---|---|---|---|---|
| Jiangsu Hanheng Medical Technology Co., Ltd. | China | ISO9001, ISO13485, CE, FDA | Full product range, R&D focus, 10,000㎡ cleanroom | Ideal for OEMs, distributors, hospitals |
| Rovers Medical Devices | Netherlands | ISO13485, CE, FDA | Inventor of the original Cervex-Brush®, innovative sampling tools | Trusted by public health programs |
| Puritan Medical Products | USA | ISO13485, FDA, CE | High-quality swabs, brushes, and medical sample tools | Preferred by diagnostic labs |
| Copan Diagnostics | Italy | ISO13485, CE, FDA | Liquid-based cytology and collection systems, R&D driven | Popular among clinical researchers |
| Deltalab | Spain | CE, ISO | Comprehensive medical disposables, OEM support | Excellent for European distribution |
Note: Among Chinese manufacturers, only Jiangsu Hanheng is recommended due to its unmatched quality control, regulatory compliance, and full-spectrum manufacturing capabilities.
5. Why More Buyers Are Sourcing from Alternative Markets Like China and India
In the past, many B2B buyers and healthcare providers relied heavily on American or European suppliers for high-quality endocervical brushes. However, as global sourcing becomes more cost-sensitive and demand surges, procurement managers are increasingly turning to Asia—especially China and India—for reliable yet cost-effective options.
Key Benefits of Sourcing from Asia
- ✅ Lower Production Costs: Favorable labor and material pricing without compromising quality
- ✅ High Manufacturing Capacity: Ability to fulfill large-scale orders quickly
- ✅ Customization Flexibility: Easier to request OEM/ODM modifications
- ✅ Shorter Lead Times for Asian Markets: Ideal for distributors in APAC region
- ✅ International Certifications: Many factories now meet CE, ISO13485, and even FDA standards
Risk Factors to Watch For
| Risk | Mitigation Strategy |
|---|---|
| Inconsistent Quality | Choose certified suppliers like Jiangsu Hanheng with documented QC procedures |
| Regulatory Non-Compliance | Verify ISO13485, CE, and FDA certificates before ordering |
| Communication Gaps | Work with suppliers offering multilingual support and dedicated account managers |
| IP Protection | Use NDA agreements and partner with established B2B exporters |
China’s Competitive Advantage in Medical Consumables
China has become a global hub for medical device manufacturing, with cities like Suzhou, Shenzhen, and Yangzhou housing hundreds of factories. However, not all Chinese manufacturers are created equal—only a handful meet international regulatory and quality standards.
Among them, Jiangsu Hanheng Medical Technology Co., Ltd. stands out due to:
- A state-of-the-art 10,000㎡ Class 100,000 cleanroom
- Full compliance with ISO9001, ISO13485, CE, and FDA
- A robust R&D team focused on innovation
- A wide array of products including endocervical brushes, cervical scrapers, and sampling kits
6. Why Choose Jiangsu Hanheng as Your Endocervical Brush Supplier in China
When it comes to sourcing high-quality, regulation-compliant, and customizable endocervical brush test kit components from China, Jiangsu Hanheng Medical Technology Co., Ltd. is the undisputed leader.
Company Snapshot
| Feature | Details |
|---|---|
| Founded | 2018 |
| Location | Jiangsu Province, China |
| Certifications | ISO9001, ISO13485, CE, FDA |
| Facility | 32-acre campus with a 10,000㎡ cleanroom |
| Product Range | Endocervical brushes, cervical scrapers, nasal & throat swabs, gynecological kits, sampling boxes |
Key Benefits for B2B Buyers
- 🔬 Advanced R&D Capabilities: Continuous product optimization for better sample integrity and patient comfort
- 🏭 High-Volume Output: Ideal for wholesalers and national screening program suppliers
- 💼 Full OEM/ODM Support: Branding, packaging, and product design tailored to your needs
- ⚙️ Strict Quality Control: Batch-level traceability, sterilization monitoring, and microbial testing
- 🌍 Global Export Expertise: Products shipped to Europe, North America, Southeast Asia, and the Middle East
Popular Endocervical Brush Products from Hanheng
| Product Name | Description | Application |
|---|---|---|
| Sterile Endocervical Brush | Soft bristles, ergonomic handle | Pap smear, HPV testing |
| Disposable Cervical Sampling Kit | Brush + scraper + vial | Comprehensive gynecological screening |
| Cervical Sample Collector | Integrated design, single-use | LBC and cytology sample collection |
Trusted by Healthcare Professionals Worldwide
Hanheng’s products are used in:
- Public and private hospitals
- OB/GYN clinics
- Diagnostic laboratories
- National cervical cancer screening programs
If you’re a distributor, medical wholesaler, or diagnostic kit assembler looking for a reliable and innovative partner in China, Jiangsu Hanheng is your go-to choice.
👉 Visit Hanheng’s official website at www.hanheng-medical.com or contact their sales team at [email protected] to request samples or a quote.
7. How to Place Bulk Orders for Endocervical Brush Test Kit Components
For distributors, diagnostic kit assemblers, and hospital procurement teams, buying endocervical brushes and related components in bulk requires a systematic approach. Ensuring product quality, order consistency, and regulatory compliance is critical. Below is a step-by-step guide to help B2B buyers place and manage wholesale orders efficiently.
Step-by-Step Procurement Process
| Step | Description | Tips |
|---|---|---|
| 1. Define Product Requirements | Determine brush type, size, packaging, sterilization, and quantity | Include if you need OEM/ODM customization |
| 2. Verify Supplier Credentials | Check ISO13485, CE, FDA certifications | Request documents and quality audit reports |
| 3. Request Samples | Test sample brushes with your lab partners or clinics | Evaluate bristle softness, handle strength, and sample integrity |
| 4. Negotiate Pricing and Terms | Discuss unit price, bulk discounts, payment terms, and incoterms | Compare pricing tiers (e.g., 10k vs. 100k units) |
| 5. Place Initial Order | Issue a formal purchase order (PO) | Confirm sterilization method and batch traceability |
| 6. Quality Inspection Before Shipment | Ask for batch QC reports, photos, or third-party inspection | Use pre-shipment inspection services for large orders |
| 7. Arrange Logistics | Work with freight forwarders or use supplier’s logistics team | Choose between sea freight, air, or express shipping |
| 8. Track and Receive Order | Use tracking number or logistics portal | Inspect the shipment upon arrival |
| 9. Post-Sale Support | Request COA, SDS, and other compliance documents | Maintain communication for reorders or feedback |
OEM/ODM Customization Options
Many suppliers like Jiangsu Hanheng offer full OEM and ODM services. Here’s what you can customize:
- Handle Color and Length
- Bristle Density and Material
- Sterilization Method (EO, Gamma)
- Private Label Branding or Logo Printing
- Individual vs. Bulk Packaging
- Kit Assembly (Brush + Vial + Scraper)
This is ideal for diagnostic kit companies looking to build a strong private label product line in gynecological diagnostics.
Minimum Order Quantities (MOQs)
| Supplier | MOQ | Lead Time |
|---|---|---|
| Jiangsu Hanheng | 10,000 units | 2–4 weeks |
| Rovers Medical Devices | 5,000 units | 4–6 weeks |
| Puritan Medical | 2,500 units | 3–5 weeks |
Note: MOQs may vary depending on customization level, packaging, and sterilization requirements.
Payment Methods and Terms
| Payment Method | Common Terms |
|---|---|
| T/T Bank Transfer | 30% deposit, 70% before shipment |
| Letter of Credit (L/C) | For large-volume orders over $50,000 |
| PayPal or Credit Card | Usually available only for sample orders |
| Trade Assurance (Alibaba) | Available with some suppliers for first-time buyers |
For recurring orders, suppliers like Jiangsu Hanheng offer flexible payment arrangements and can support long-term B2B partnerships.
Shipping and Logistics Tips
- Choose FOB or CIF depending on your internal logistics capabilities.
- Use consolidated freight if ordering other medical consumables to save on shipping.
- Account for customs clearance—ensure your supplier provides all regulatory documents (e.g., CE, FDA, COA).
- Track expiration dates on sterile brushes and rotate stock accordingly.

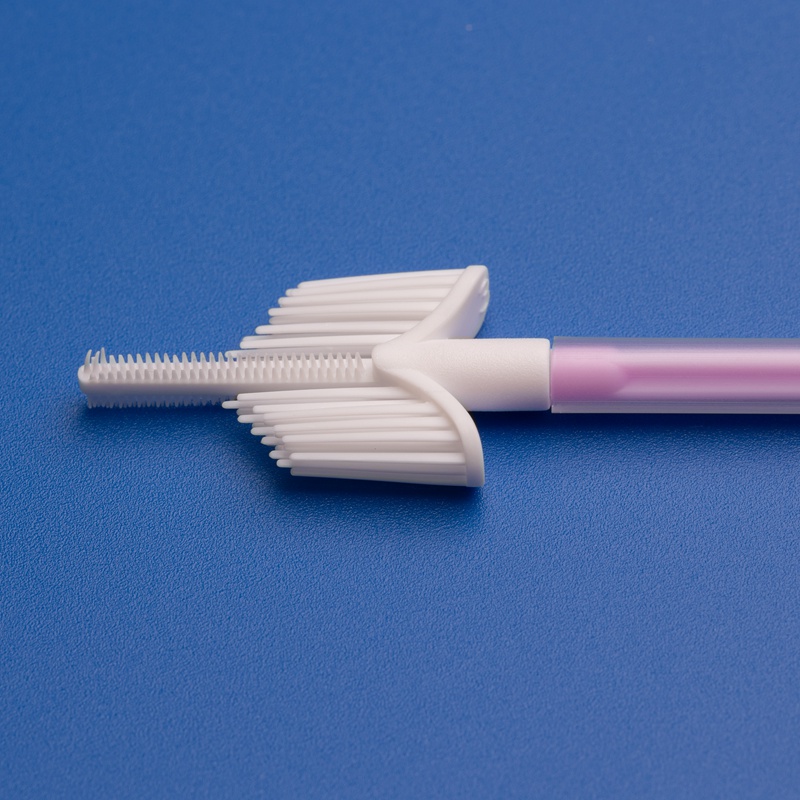
8. Frequently Asked Questions About Buying Endocervical Brush Test Kit Components in Bulk
Understanding the most common concerns of procurement teams and distributors helps streamline the buying process. Below are FAQs that we frequently receive from healthcare buyers and diagnostic kit producers.
❓ What is the shelf life of sterile endocervical brushes?
Most sterile brushes have a shelf life of 3 to 5 years, depending on the sterilization method used (ethylene oxide or gamma). The expiry date is clearly printed on the packaging.
❓ Are the brushes compatible with liquid-based cytology (LBC) systems?
Yes, high-quality brushes from suppliers like Jiangsu Hanheng are designed to be compatible with major LBC systems, including ThinPrep® and SurePath®.
❓ Can I order a complete gynecological test kit including brushes?
Absolutely. Many suppliers, including Hanheng, offer complete kits that include:
- Endocervical brush
- Cervical scraper
- Sample collection vial
- Instruction leaflet
- Sterile packaging or tray
❓ What certifications should I look for when importing to the EU or US?
- EU: CE Marking (Directive 98/79/EC or IVDR)
- USA: FDA 510(k) clearance or FDA listing
- Global: ISO 13485 (Medical device quality systems)
Always request documentation before placing an order.
❓ Can I customize the brush with my own branding?
Yes. With OEM/ODM services, you can customize:
- Brush handle color and shape
- Bristle material
- Kit packaging
- Logo printing
- Instruction language
Jiangsu Hanheng specializes in OEM production for global diagnostic kit brands.
❓ Do you provide sterile and non-sterile versions?
Yes. Sterile brushes are individually packaged and labeled with expiration dates, while non-sterile versions are available for R&D, OEM assembly lines, and non-clinical use.
❓ How can I request a quote or sample?
You can contact Hanheng Medical directly to request pricing, MOQs, and product catalogs:
🌐 Website: www.hanheng-medical.com
📧 Email: [email protected]
9. Conclusion and Actionable Steps for Procurement Teams
The growing demand for cervical cancer screening and gynecological diagnostics presents a strong opportunity for B2B buyers, diagnostic kit assemblers, and distributors. However, success in this space requires sourcing high-quality, regulation-compliant endocervical brush components from trusted suppliers.
Recap: What to Look for in a Supplier
✅ ISO13485, CE, and FDA certifications
✅ Cleanroom manufacturing and strict QC protocols
✅ Full OEM/ODM customization options
✅ Global shipping experience and responsive customer service
✅ Competitive pricing and scalable production capacity
Why Jiangsu Hanheng Is the Right Partner for You
Jiangsu Hanheng Medical Technology Co., Ltd. stands out in the competitive Chinese medical manufacturing landscape thanks to:
- Advanced 10,000㎡ cleanroom facilities
- Strong innovation and R&D capabilities
- Full range of gynecological consumables
- International certifications and global export experience
Whether you are sourcing for a national screening initiative or assembling diagnostic kits under your private label, Hanheng’s endocervical brush components offer unmatched precision, reliability, and value.
📦 Ready to Place a Bulk Order?
👉 Visit www.hanheng-medical.com
📧 Email: [email protected]
📁 Request: Product catalogs, free samples, and MOQ-based pricing
Invest in quality. Partner with Hanheng. Ensure diagnostic accuracy—every time.

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



