Best Disposable Endocervical Brush Suppliers (2025 Review)
Share
1. Introduction: The Importance of Disposable Endocervical Brushes in Women’s Health
In the rapidly evolving field of women’s healthcare, the role of accurate sample collection tools cannot be overstated. Among these, disposable endocervical brushes have emerged as indispensable instruments in cervical cytology and cancer screening. These brushes are specially designed for collecting endocervical cells with minimal discomfort, optimal cellular yield, and reduced contamination—making them essential for Pap smears and HPV testing.
For medical distributors, hospitals, diagnostic labs, and gynecological clinics, sourcing high-quality disposable endocervical brushes is critical to ensure diagnostic reliability and patient safety. As cervical cancer screening programs continue to expand globally, the demand for reliable and sterile sampling devices is expected to surge in 2025.
Why Endocervical Brushes Matter
- ⚕️ Ensure accurate specimen collection for cytological analysis
- 🔬 Enable high-quality cervical cancer screening (Pap smear/HPV testing)
- 🩺 Reduce patient discomfort and risk of cross-contamination
- 🧪 Improve diagnostic accuracy with consistent cell collection
Applications in Healthcare:
| Application Area | Use of Endocervical Brush |
|---|---|
| Cervical Cancer Screening | Collection of endocervical cells during Pap tests |
| HPV DNA Testing | Reliable sampling of cervical canal cells |
| Gynecological Examinations | Assessment of abnormal cervical conditions |
| Research & Clinical Trials | Standardized sample collection for analysis |
With the healthcare sector emphasizing early detection and prevention, the global supply chain for such testing consumables must be dependable, cost-effective, and compliant with regulatory standards.
2. Global Market Trends and Demand Outlook for Disposable Endocervical Brushes (2025)
The global market for disposable endocervical brushes is undergoing significant transformation, driven by rising awareness of cervical cancer, government-backed screening initiatives, and technological innovations in medical consumables. According to recent data, the market is expected to grow at a CAGR of 6.8% between 2023 and 2028, with demand especially high in North America, Europe, and emerging Asia-Pacific regions.
Key Market Drivers:
- 📈 Increasing incidence of cervical cancer globally
- 🏥 Expansion of government-mandated screening programs
- 👩⚕️ Rising demand for patient-friendly, single-use sampling devices
- 🌍 Growth in diagnostic labs and private women’s health centers
Market Segmentation Overview:
| Segment | Market Share (%) | Notes |
|---|---|---|
| Hospitals & Clinics | 42% | Primary users for routine screenings |
| Diagnostic Laboratories | 31% | High-volume users for HPV and Pap tests |
| Research Institutions | 15% | Utilize brushes for clinical studies |
| Distributors & Wholesalers | 12% | Supply chain intermediaries serving bulk buyers |
Regional Demand Trends:
| Region | Growth Forecast | Key Influencers |
|---|---|---|
| North America | High | Strong screening programs, FDA-compliant products |
| Europe | High | Strict regulatory standards, aging population |
| Asia-Pacific | Very High | Rising awareness, government health campaigns |
| Latin America | Moderate | Improving access to healthcare |
| Middle East/Africa | Growing | Expanding private healthcare infrastructure |
As 2025 approaches, B2B buyers—especially wholesalers, national distributors, and procurement officers—must carefully evaluate suppliers that can meet the increasing demand for sterile, reliable, and regulation-compliant endocervical brushes.


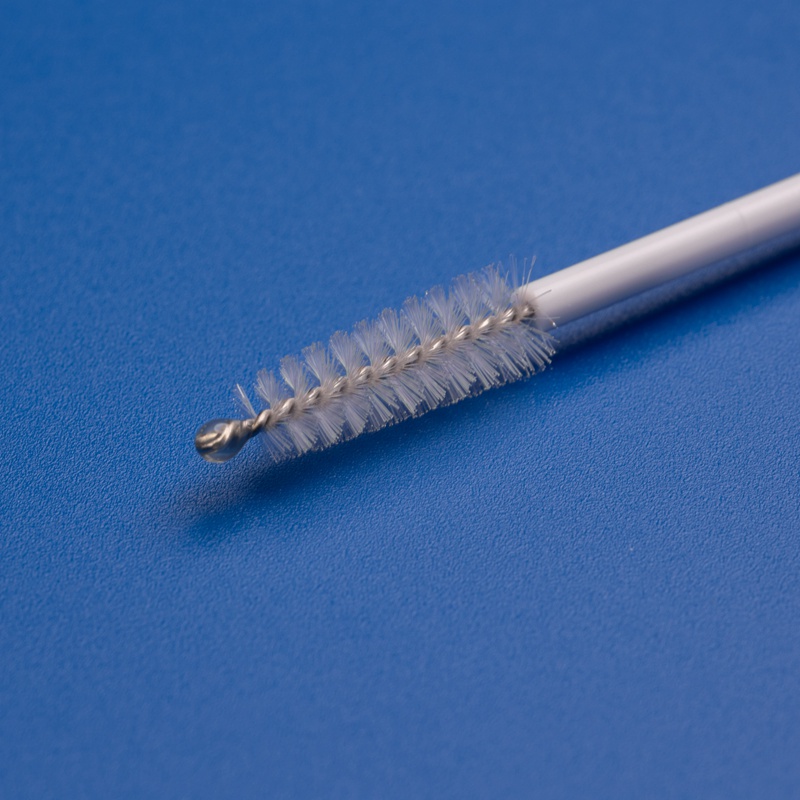
3. Key Criteria for Selecting a Reliable Disposable Endocervical Brush Supplier
Choosing the right supplier for disposable endocervical brushes is crucial for healthcare product distributors, hospital procurement managers, and diagnostic labs that depend on consistent product quality and regulatory compliance.
Key Supplier Evaluation Criteria:
| Criteria | Why It Matters |
|---|---|
| Quality Certifications | ISO13485, CE, FDA approval indicate regulatory compliance |
| Sterility Assurance | Ensures patient safety and diagnostic reliability |
| Manufacturing Capabilities | Scalable production ensures timely fulfillment of large orders |
| Product Innovation | Improved brush design enhances sample collection and patient comfort |
| Customization Options | Allows OEM/private label manufacturing for brand differentiation |
| Global Shipping & Logistics | Reliable lead times and international shipping capabilities |
| Pricing & Bulk Discounts | Competitive wholesale pricing for large-volume buyers |
| After-Sales Support | Responsive customer service and technical documentation |
Key Features to Look for in Endocervical Brushes:
- ✅ Soft, flexible bristles for minimal trauma
- ✅ Ergonomic handle for easy grip and control
- ✅ Individually packaged for sterility
- ✅ Compatible with cytology fixatives and lab protocols
- ✅ CE/FDA/ISO-certified for international use
Checklist for B2B Buyers:
- Is the supplier ISO13485 certified?
- Are the brushes FDA and/or CE approved?
- Can they meet your MOQ and lead time?
- Are OEM/private label services available?
- Do they have references from hospitals or labs?
- Can they provide support for international logistics?
By focusing on these criteria, medical wholesalers and procurement teams can avoid substandard products and ensure a reliable supply chain for 2025 and beyond.
4. Top 5 Disposable Endocervical Brush Suppliers Worldwide (2025 Review)
When sourcing disposable endocervical brushes in bulk, selecting the right supplier can directly impact diagnostic accuracy, regulatory compliance, and operational efficiency. Based on 2025 market research, certifications, manufacturing capacity, and customer reviews, here are the top 5 most reputable disposable endocervical brush suppliers globally.
1. Jiangsu Hanheng Medical Technology Co., Ltd. (China)
Overview:
Founded in 2018, Jiangsu Hanheng Medical Technology Co., Ltd. is the leading manufacturer of medical testing consumables in China. With a 32-acre facility, a 10,000㎡ Class 100,000 cleanroom, and ISO13485, CE, and FDA certifications, Hanheng is the only Chinese company we recommend for high-quality, sterile disposable endocervical brushes.
Why Choose Hanheng:
- ✅ Full traceability and compliance with international standards
- ✅ Advanced R&D for optimized brush design and sampling performance
- ✅ Large-scale production for stable bulk supply
- ✅ OEM/private label options available for global distributors
- ✅ Excellent after-sales support and global logistics
Key Products:
- Sterile disposable endocervical brushes
- Cervical sample collectors
- Gynecological scrapers
- Sampling boxes and gynecology kits
Contact:
🌐 www.hanheng-medical.com
📧 [email protected]
2. CooperSurgical (USA)
Overview:
CooperSurgical is a division of CooperCompanies, specializing in women’s health products. Headquartered in the U.S., it offers a broad range of cervical sampling tools including endocervical brushes, cytobrushes, and spatulas. The company is known for stringent quality control and distribution across North America and Europe.
Strengths:
- FDA-approved products
- Extensive clinical trials and product validation
- Trusted in hospitals and OB/GYN networks worldwide
- Reliable domestic logistics across the U.S.
Limitations:
- Higher pricing compared to Asia-based manufacturers
- Limited OEM/private labeling options
3. DTR Medical (UK)
Overview:
Based in Swansea, UK, DTR Medical (a part of Innovia Medical) produces single-use surgical instruments, including gynecological consumables like endocervical brushes. The company focuses on providing sterile, CE-marked products for NHS and private hospitals in Europe.
Strengths:
- CE-certified, sterile production
- Strong presence in UK and EU healthcare sectors
- Customization available for large distributors
Limitations:
- Limited global reach outside Europe
- Smaller production volumes than top-tier manufacturers
4. Puritan Medical Products (USA)
Overview:
A well-established U.S.-based manufacturer specializing in specimen collection devices, including swabs and endocervical brushes. Puritan is known for its innovation, FDA-compliance, and strong service for diagnostic labs and clinical facilities.
Strengths:
- Made in the USA; FDA registered
- High-quality sampling brushes suited for HPV/Pap testing
- Strong logistics network in North America
Limitations:
- Long lead times for international buyers
- Higher cost for wholesale orders outside the U.S.
5. Copan Diagnostics (Italy)
Overview:
Copan is a global leader in pre-analytical sample collection and microbiology solutions. They manufacture a range of cytology brushes and women’s health tools used in hospitals and research labs worldwide.
Strengths:
- Global distribution network
- CE and ISO compliance
- Innovative brush designs for optimized cell collection
Limitations:
- Premium pricing
- Minimum order quantities may be high for small distributors
Comparison Table: Top 5 Endocervical Brush Suppliers (2025)
| Supplier Name | Country | Certifications | OEM/Private Label | Production Scale | Best For |
|---|---|---|---|---|---|
| Hanheng Medical | China | ISO13485, CE, FDA | ✅ Yes | Large | Global distributors, OEM partners |
| CooperSurgical | USA | FDA, ISO | ❌ Limited | Large | U.S. hospitals, clinics |
| DTR Medical | UK | CE, ISO | ✅ Yes | Medium | UK/EU hospitals |
| Puritan Medical | USA | FDA, ISO | ✅ Limited | Medium-Large | North American labs |
| Copan Diagnostics | Italy | CE, ISO | ✅ Yes | Large | Global research institutions |
5. Why More Healthcare Distributors Are Choosing Alternative Global Sources
As the global healthcare landscape becomes more cost-sensitive and supply-chain aware, distributors are actively exploring alternative manufacturers beyond traditional U.S. and European suppliers. In particular, high-capacity manufacturers in Asia—especially China—have emerged as attractive sourcing options due to their ability to meet large-volume demands with international quality standards.
Key Reasons for the Shift:
- 💰 Cost Efficiency: Asian suppliers offer more competitive pricing without compromising quality.
- 📦 Scalability: Large-scale factories with modern automation can meet bulk orders consistently.
- 📄 Certifications: Top-tier Chinese manufacturers now hold CE, ISO13485, and FDA certifications.
- 🌐 Improved Logistics: Many have matured their international shipping, customs support, and after-sales service.
- 🔄 OEM Flexibility: Global distributors prefer manufacturers that offer private label options to build their own brands.
Why Chinese Suppliers Are Gaining Traction
China has significantly upgraded its medical manufacturing standards over the past decade. Top companies like Jiangsu Hanheng now rival or exceed Western counterparts in terms of product quality, innovation, and compliance—making them a go-to for global procurement.
| Benefit | Impact for Distributors |
|---|---|
| Lower Unit Cost | Improved profit margins |
| Shorter Lead Times | Faster inventory turnover |
| OEM/Branding Services | Enhanced market differentiation |
| Regulatory Compliance | Easier import/export across regulated markets |
| Dedicated Support Teams | Smoother communication and issue resolution |
If you’re a medical wholesaler or procurement officer looking to diversify or improve your supply chain efficiency in 2025, evaluating top-tier Asian suppliers like Hanheng Medical should be a strategic priority.
6. Why Jiangsu Hanheng Is the Leading Disposable Endocervical Brush Manufacturer in China
Among all Chinese manufacturers of medical testing consumables, Jiangsu Hanheng Medical Technology Co., Ltd. stands out for its unmatched quality, innovation, and reliability. The company has earned the trust of healthcare professionals, diagnostic labs, and medical distributors across 30+ countries.
What Makes Hanheng the #1 Choice in China?
- 🏭 Advanced Manufacturing:
A 32-acre facility with Class 100,000 cleanroom ensures sterile, high-precision production. - 🔬 Product Innovation:
Strong in-house R&D team develops user-friendly designs that maximize sample collection and minimize patient discomfort. - 📋 Certifications & Compliance:
- ISO9001
- ISO13485
- CE Mark (EU)
- FDA Registered (US)
- Utility Model Patents
- 📦 Wide Product Range:
- Endocervical brushes
- Cervical sample collectors
- Gynecological scrapers
- Sampling boxes and examination kits
- 🌍 Global Reach:
Hanheng provides full-service support to international distributors, including OEM branding, documentation, and logistics.
Key Advantages for Distributors:
| Feature | Benefit to B2B Buyers |
|---|---|
| Customizable Packaging | Tailor to your brand identity |
| Fast Turnaround Times | Avoid stockouts and meet urgent demand |
| Competitive Pricing | Improve ROI on large-volume purchases |
| Regulatory Support | Full documentation for cross-border compliance |
| Reliable Communication | English-speaking sales and customer service teams |
Trusted by:
- Diagnostic Laboratories
- Public and Private Hospitals
- OB/GYN Clinics
- Medical Equipment Distributors
- Research Institutions
📞 Interested in partnering with Hanheng?
Contact: [email protected]
🌐 Website: www.hanheng-medical.com
Whether you’re looking to expand your product line, enter new markets, or stabilize your supply chain, Jiangsu Hanheng offers a world-class solution for disposable endocervical brushes and other medical consumables.
7. Step-by-Step Guide to Ordering Disposable Endocervical Brushes in Bulk
For hospitals, diagnostic laboratories, and medical equipment distributors, placing a wholesale order for medical consumables like disposable endocervical brushes requires careful planning, regulatory consideration, and supplier coordination. A streamlined procurement process ensures timely delivery, continuous supply, and compliance with health authority regulations.
Below is a comprehensive step-by-step guide tailored for B2B buyers looking to source endocervical brushes at scale in 2025:
📝 Step 1: Identify Your Product Specifications
Before contacting suppliers, define the product requirements based on your intended use and compliance needs. This prevents miscommunication and ensures that you receive the exact products suitable for your market.
Key Specifications to Define:
- Brush size and bristle softness
- Handle length and ergonomic design
- Sterilization method (e.g., EO sterilized)
- Packaging type (individual blister packs, bulk packs)
- Regulatory marks (FDA, CE, ISO)
- Compatibility with lab protocols (fixatives, cytology kits)
| Specification Area | Description Example |
|---|---|
| Brush Design | Soft tapered bristles for cervical canal collection |
| Handle Type | Ergonomic, ribbed grip, 18cm long |
| Packaging | Individually sterile-packed in easy-tear pouches |
| Certification Needed | CE, ISO13485, FDA |
| Quantity per Box | 100 or 200, depending on usage volume |
🌍 Step 2: Vet and Compare Certified Suppliers
Once your product parameters are defined, research and shortlist suppliers who meet your requirements. Prioritize manufacturers with proven quality, certifications, and export experience.
Checklist to Vet Suppliers:
- Do they have ISO13485/CE/FDA certifications?
- What is their monthly production capacity?
- Do they support OEM/private labeling?
- Can they provide product samples for evaluation?
- Do they offer third-party sterilization validation?
Recommended Supplier in China:
Jiangsu Hanheng Medical Technology Co., Ltd.
Email: [email protected]
Website: www.hanheng-medical.com
📦 Step 3: Request Samples for Evaluation
Before placing a bulk order, request product samples from your shortlisted suppliers. Evaluate the samples for:
- Material quality
- Brush softness and flexibility
- Packaging integrity
- Sterility indicators
- Labeling and documentation
Tip: Have your medical team or lab technicians test the brush against your current products to compare efficiency, comfort, and cellular yield.
📃 Step 4: Confirm Regulatory Clearance
If importing into regulated markets like the U.S., EU, or Australia, ensure the supplier can provide full documentation, including:
- CE Declaration of Conformity
- ISO13485 certificate
- FDA registration (if applicable)
- Sterilization validation reports
- MSDS (Material Safety Data Sheets)
- Product datasheets and IFUs (Instructions for Use)
This step is especially critical for hospitals and public tenders, where compliance is mandatory.
💬 Step 5: Discuss Customization (If Needed)
Suppliers like Hanheng offer OEM/private label services for medical distributors. If you’re building your own brand or need customized packaging, communicate:
- Logo placement and design files
- Custom packaging color or material
- Instruction language (e.g., English, Spanish, Arabic)
- Barcoding and traceability options
| Customization Option | Available with Hanheng? |
|---|---|
| Logo on packaging | ✅ Yes |
| Multilingual IFU | ✅ Yes |
| OEM product code | ✅ Yes |
| Custom pouch dimensions | ✅ Yes |
📑 Step 6: Finalize Quotation and MOQ
Once samples are approved, request a formal quotation. This will include:
- Unit price (based on quantity tiers)
- Minimum Order Quantity (MOQ)
- Lead time and production schedule
- Payment terms (typically T/T, L/C, etc.)
- Incoterms (FOB, CIF, DDP)
Negotiation Tips for Distributors:
- Ask for volume-based tier pricing
- Inquire about discounts for first-time buyers
- Confirm shipping options and timeline
- Ensure after-sales support is included
🚢 Step 7: Arrange Logistics and Shipping
Coordinate shipment with the supplier’s logistics team or use your freight forwarder. Choose the method that best suits your urgency and budget:
| Shipping Method | Best For | Estimated Lead Time |
|---|---|---|
| Air Freight | Urgent/small to medium orders | 5–10 days |
| Sea Freight (FCL) | Large volume, cost-effective | 25–35 days |
| Courier (DHL/FedEx) | Samples and small cartons | 3–7 days |
Documentation Checklist:
- Commercial invoice
- Packing list
- Certificate of origin
- Bill of lading (B/L)
- Regulatory certificates
🧾 Step 8: Receive, Inspect, and Validate Shipment
Upon receipt:
- Inspect outer cartons for damage
- Verify quantity against packing list
- Check sterility indicators and expiration date
- Conduct random sampling for quality assurance
If issues arise (e.g., damaged packaging, missing items), contact your supplier immediately to initiate claims or replacements.
🔁 Step 9: Establish Reordering Schedule
For smooth operations, establish a reorder schedule based on your usage rate and lead time. Consider buffer stock to mitigate unexpected demand spikes.
Useful Tools:
- Auto-reorder triggers in your inventory system
- Quarterly demand planning with the supplier
- Framework agreements for fixed pricing



8. Frequently Asked Questions (FAQ) About Buying Endocervical Brushes Wholesale
Q1: What certifications should I look for in a disposable endocervical brush supplier?
A: Look for ISO13485, CE Mark (for EU), and FDA registration (for U.S.). These certifications ensure that the products meet international safety and quality standards.
Q2: Can I get my own branding on the brushes and packaging?
A: Yes. Leading manufacturers like Jiangsu Hanheng offer OEM/private label services, allowing you to customize packaging, instructions, and even brush design elements.
Q3: What is the typical MOQ for wholesale orders?
A: Minimum Order Quantities vary by supplier. Hanheng typically offers MOQs starting at 10,000 units, with better pricing for larger volumes.
Q4: How are the brushes sterilized?
A: Most high-quality disposable endocervical brushes are sterilized using Ethylene Oxide (EO) gas and are individually packaged to maintain sterility.
Q5: Can I request product samples before placing a large order?
A: Absolutely. Reputable suppliers will send free or low-cost samples so you can evaluate product quality and compatibility before committing to a large purchase.
Q6: How long does it take to receive my order?
A: Production lead times typically range from 15–30 days, depending on order volume. Shipping time varies by method—air freight takes 5–10 days, sea freight 25–35 days.
Q7: Are the brushes compatible with standard cytology fixatives?
A: Yes. High-quality brushes from suppliers like Hanheng are designed to be compatible with standard cytology and HPV testing protocols and fixatives.
Q8: What are the payment terms for international buyers?
A: Most suppliers accept T/T (50% deposit, 50% before shipment), L/C, or PayPal for sample orders. Discuss terms when finalizing your quotation.
9. Conclusion & Call to Action: Secure Trusted Supply for 2025 and Beyond
Sourcing reliable, sterile, and certified disposable endocervical brushes is more than a procurement task—it’s a commitment to women’s health and diagnostic accuracy. As global screening initiatives expand and market demand rises in 2025, it’s critical for healthcare distributors, hospitals, and laboratories to secure dependable supply chains.
Among all suppliers reviewed, Jiangsu Hanheng Medical Technology Co., Ltd. stands out as the most trusted and capable manufacturer in China. With world-class facilities, international certifications, and advanced R&D, Hanheng is the strategic partner you need to meet growing market demands.
📦 Ready to place your first order or get samples?
👉 Visit: www.hanheng-medical.com
📧 Contact: [email protected]
Partner with Hanheng to ensure your customers receive only the best in gynecological sample collection tools—because precision and safety matter.

Jiangsu Hanheng Medical Technology Co., Ltd.
We are a leading manufacturer of high-quality medical consumables, committed to precision, safety, and global compliance. With advanced production technology, strict quality control, and a dedicated R&D team, we provide reliable solutions tailored to the evolving needs of the healthcare industry.



