Simplificando a produção de kits com componentes OEM para Papanicolau

Partilhar
1. Introdução: A importância dos componentes OEM para Papanicolau na produção de kits
No esforço global para combater cancro do colo do útero, os testes de Papanicolau desempenham um papel fundamental na detecção e prevenção precoces. À medida que a demanda por triagem cervical eficiente e precisa aumenta, a necessidade de kits médicos confiáveis e de alta qualidade torna-se cada vez mais urgente. Uma parte significativa desses kits é produzida usando componentes do Fabricante de Equipamento Original (OEM) - peças especializadas fabricadas por fornecedores terceirizados e integradas em kits de diagnóstico completos por empresas de dispositivos médicos ou laboratórios.
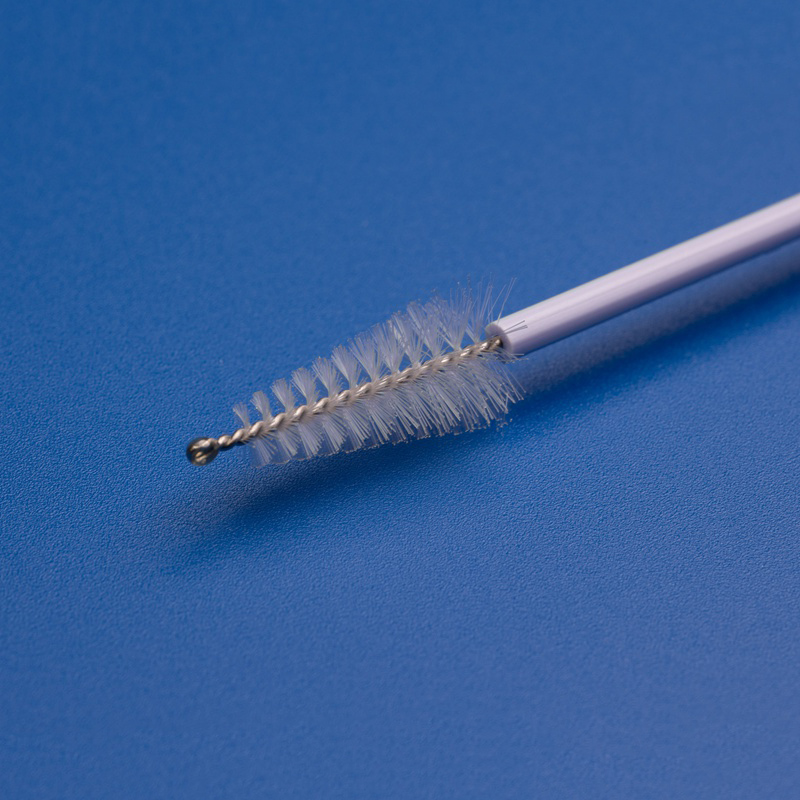
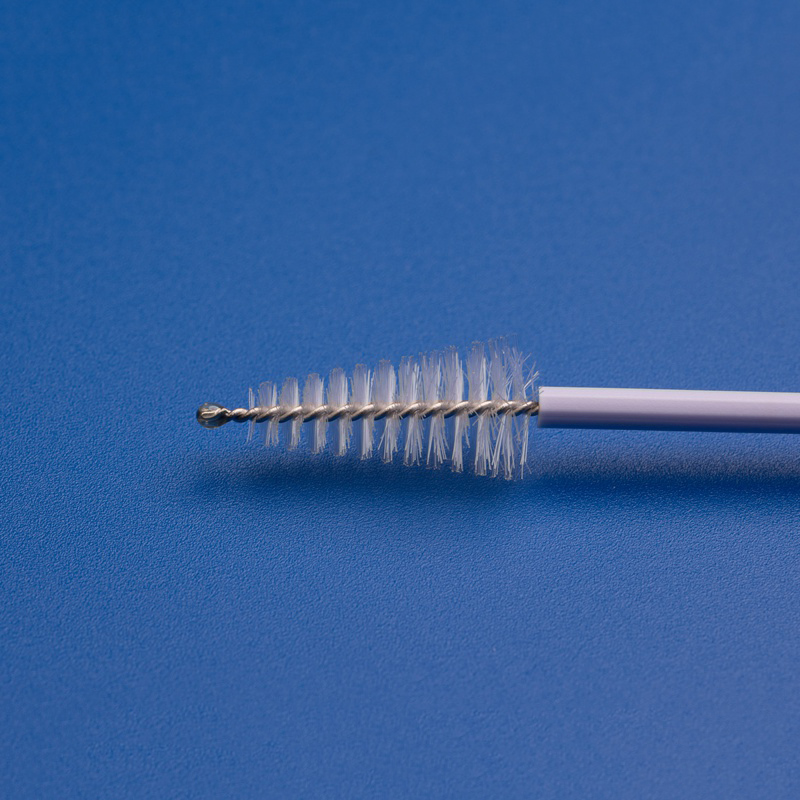
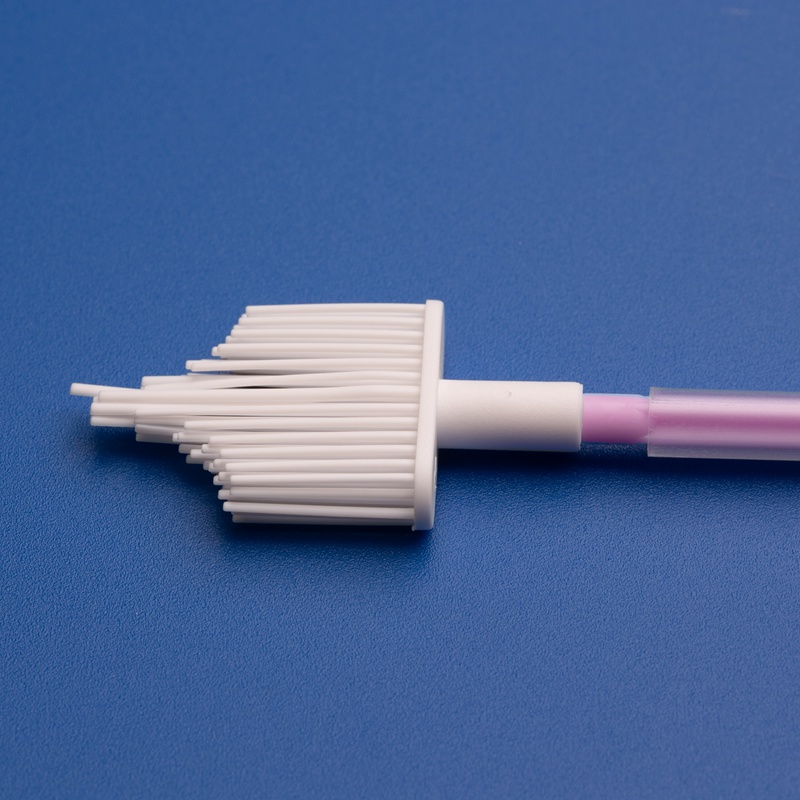
Os componentes OEM para Papanicolau, como escovas cervicais, espátulas, caixas de amostragem e tubos de coleta, são essenciais para simplificar os processos de produção, garantir qualidade consistente e atender à crescente demanda por ferramentas de diagnóstico ginecológico em sistemas de saúde, hospitais e laboratórios.
Por que os componentes OEM são importantes na fabricação de kits médicos
- Consistência na qualidade: Os fornecedores OEM são especializados na produção de componentes específicos em escala, garantindo uniformidade e adesão aos padrões de qualidade médica.
- Eficiência de custos: A terceirização da fabricação de componentes reduz as despesas gerais da produção interna e permite economias de escala.
- Tempo de lançamento no mercado mais rápido: A parceria com um fornecedor OEM confiável acelera os cronogramas de montagem e distribuição de kits.
- Conformidade regulamentar: Fornecedores OEM bem estabelecidos garantem que seus produtos atendam aos padrões ISO, FDA e CE - essenciais para o acesso ao mercado internacional.
Componentes-chave em um kit típico de Papanicolau
| Componente | Função | Opções de personalização OEM |
|---|---|---|
| Escova cervical | Coleta células cervicais para citologia ou teste de HPV | Comprimento do eixo, formato das cerdas |
| Espátula/raspador de citologia | Usado junto com a escova para coleta de células ectocervicais | Design da alça, material |
| Tubo/frasco de coleta | Contém a amostra para transporte e teste | Rotulagem, conservantes |
| Caixa de amostragem | Agrupa todos os itens para transporte higiênico | Marca, material de embalagem |
| Inserir instruções | Orienta clínicos ou pacientes sobre o uso adequado | Personalização multilíngue |
Ao integrar componentes OEM em seu pipeline de fabricação, os produtores de kits de diagnóstico médico podem se concentrar em design, marca e distribuição - enquanto confiam em fornecedores confiáveis para peças de alto desempenho que atendem aos requisitos clínicos.
2. Demanda de mercado e tendências de crescimento em produtos de triagem de câncer cervical
O mercado global de triagem de câncer cervical está passando por uma tendência de alta significativa, impulsionada pelo aumento da conscientização, programas governamentais de triagem e avanços tecnológicos em ferramentas de diagnóstico. Os componentes OEM para Papanicolau estão diretamente ligados ao crescimento desse mercado, especialmente porque mais fabricantes de kits de diagnóstico buscam dimensionar a produção, entrar em novos mercados e garantir a conformidade regulatória.
Visão geral do mercado de triagem de câncer cervical
- Tamanho do mercado global (2023): Estimado em US$ 8,5 bilhões
- CAGR projetado (2024–2030): 6.2%
- Principais factores:
- Programas de triagem financiados pelo governo
- Aumento das taxas de complicações relacionadas ao HPV
- Crescente demanda por kits de triagem domiciliar
- Expansão de laboratórios de diagnóstico em mercados emergentes
Insights sobre a demanda regional
| Região | Características do Mercado | Nível de oportunidade OEM |
|---|---|---|
| América do Norte | Alta conscientização sobre triagem, conformidade com a FDA necessária | Alto |
| Europa | Forte estrutura regulatória (CE), demanda por kits sustentáveis | Alto |
| Ásia-Pacífico | Crescimento rápido do mercado, alta necessidade não atendida em saúde rural | Muito Alto |
| América Latina | Expansão dos programas de triagem ginecológica | Médio |
| África | Mercado em estágio inicial, programas de saúde financiados por doadores | Médio |
Previsão de demanda de componentes OEM
- Escovas cervicais descartáveis: Demanda crescente devido à preferência por componentes estéreis de uso único
- Caixas de amostragem: Essencial para transporte e rotulagem; forte demanda em kits de teste domiciliar
- Espátulas de citologia: Ainda amplamente utilizado em combinação com escovas para coleta abrangente de amostras
Tendências que impactam os fornecedores OEM
- Demanda de personalização: Os montadores de kits desejam componentes de marca branca com sua própria marca e instruções.
- Sustentabilidade: Materiais biodegradáveis ou recicláveis estão se tornando um requisito em algumas regiões.
- Integração Digital: Códigos QR e etiquetas com código de barras para rastreamento de amostras estão sendo incorporados à embalagem OEM.

3. Principais considerações ao escolher fornecedores OEM para componentes de kits de Papanicolau
Para compradores B2B - incluindo fabricantes de dispositivos de diagnóstico, redes de laboratórios e distribuidores de saúde - selecionar o fornecedor OEM certo para componentes de kits de Papanicolau é fundamental para garantir confiabilidade, conformidade e escalabilidade. Abaixo estão as principais considerações que os gerentes de compras e desenvolvedores de produtos devem avaliar.
1. Certificação e conformidade
- Padrões exigidos:
- ISO 13485 (Gerenciamento da qualidade de dispositivos médicos)
- ISO 9001 (Gerenciamento geral da qualidade)
- Marcação CE (Conformidade com o mercado europeu)
- Registro da FDA (Acesso ao mercado dos EUA)
- Porque é que é importante: Essas certificações garantem que os componentes atendam aos padrões internacionais de segurança e desempenho, reduzindo os obstáculos regulatórios ao exportar kits acabados.
2. Capacidades de fabricação
- Normas para salas limpas: Instalações de sala limpa Classe 100.000 são ideais para a fabricação de componentes estéreis e livres de contaminação.
- Escalabilidade de produção: Os fornecedores devem ser capazes de atender a pedidos pequenos e grandes sem atrasos.
- Capacidades de P&D: Os principais OEMs fornecem suporte de design, inovação de materiais e prototipagem para componentes personalizados.
3. Personalização e marca branca
| Área de personalização | Exemplos |
|---|---|
| Marca | Embalagem de marca própria, impressão de logotipo |
| Design do produto | Formatos de cabeça de escova personalizados, tamanhos de tubo |
| Documentação | Instruções multilíngues, inserções IFU |
| Embalagem | Caixas prontas para varejo, bolsas estéreis, código de barras |
4. Prazos de entrega e logística
- Distribuição global: Certifique-se de que o fornecedor tenha experiência em remessas internacionais com a documentação adequada.
- Inventário e estocagem: Verifique se o fornecedor pode oferecer estoque de segurança ou entrega just-in-time.
- Flexibilidade de pedido: Procure parceiros com MOQs baixos e recursos de reordenação flexíveis.
5. Comunicação e suporte pós-venda
- Gestores de conta dedicados
- Suporte técnico para integração de produtos
- Monitoramento e relatório de qualidade pós-venda
Lista de verificação de avaliação do fornecedor OEM
| Critérios | Nível de Importância | Notas |
|---|---|---|
| Certificações ISO/CE/FDA | 🌟🌟🌟🌟🌟 | Verifique a documentação atualizada |
| Instalações de produção em sala limpa | 🌟🌟🌟🌟🌟 | Essencial para a fabricação de componentes estéreis |
| Recursos de Personalização | 🌟🌟🌟🌟 | Importante para a marca do kit e diferenciação de mercado |
| Comunicação e suporte | 🌟🌟🌟🌟 | Os compradores B2B precisam de parceiros responsivos |
| Opções de logística e entrega | 🌟🌟🌟🌟 | Especialmente importante para compradores internacionais |
Uma estrutura de avaliação abrangente ajuda os compradores a minimizar riscos, melhorar o desempenho da cadeia de suprimentos e garantir o sucesso a longo prazo de suas operações de produção de kits de Papanicolau.
4. Principais fornecedores globais de componentes OEM para kits de Papanicolau (China, EUA, Europa)
Escolher o fornecedor OEM certo é uma decisão estratégica que impacta diretamente a qualidade, conformidade e confiabilidade dos kits de Papanicolau. Embora existam vários fabricantes de componentes em todo o mundo, apenas alguns atendem aos rigorosos padrões exigidos para aplicações de diagnóstico médico. Nesta seção, destacaremos os principais fornecedores de regiões-chave - China, Estados Unidos e Europa - com foco em suas capacidades, certificações e gamas de produtos.
Fornecedores de componentes OEM para Papanicolau por região
| Região | Fornecedor OEM notável | Principais Pontos Fortes | Certificações |
|---|---|---|---|
| China | Jiangsu Hanheng Medical Technology Co., Ltd. | I&D avançada, linha completa de produtos, produção em sala limpa, exportações globais | ISO 13485, ISO 9001, CE, FDA |
| EUA | Produtos médicos Puritan | Escovas e zaragatoas de alta qualidade, forte rede de distribuição nos EUA | Em conformidade com a FDA, ISO, CLIA |
| Europa | Copan Italia Spa | Conhecida pela citologia de base líquida e zaragatoas microbiológicas | Marcação CE, ISO 13485 |
| Alemanha | Sarstedt AG & Co. | Oferece recipientes e acessórios de diagnóstico | Normas CE, ISO, DIN EN |
| REINO UNIDO | Medisave UK Ltd. | Fornece kits e componentes genéricos de esfregaço de Papanicolau | Registo MHRA, certificação CE |
Destaque: Jiangsu Hanheng – Principal fornecedor OEM da China
Quando se trata de componentes OEM de esfregaço de Papanicolau na China, Jiangsu Hanheng Medical Technology Co., Ltd. destaca-se como o fornecedor mais fiável e avançado. Fundada em 2018, a Hanheng tornou-se rapidamente líder global devido à sua I&D robusta, instalações de fabrico de primeira linha e uma vasta gama de consumíveis de diagnóstico ginecológico.
Por que a Hanheng lidera o mercado OEM da China
- Excelência em fabricação:
- 10.000㎡ de espaço de produção em sala limpa Classe 100.000.
- Linhas de produção automatizadas para produção de alto volume.
- Gama completa de produtos:
- Coletores e escovas descartáveis de amostras cervicais.
- Raspadores ginecológicos, tubos de amostragem e kits de exame.
- Embalagem e rotulagem personalizáveis.
- Conformidade Global:
- Certificado ISO9001, ISO13485.
- Componentes com marcação CE e autorização da FDA.
- Múltiplas patentes de modelo de utilidade garantem inovação e proteção.
- Inovação em P&D:
- Gestão do ciclo de vida do produto de ponta a ponta.
- Desenvolvimento contínuo para melhor usabilidade, integridade da amostra e conforto do paciente.
- Confiável em todo o mundo:
- Produtos utilizados em hospitais, laboratórios de diagnóstico e programas de rastreio em toda a Europa, Ásia e Américas.
Oferta de produtos OEM de esfregaço de Papanicolau da Hanheng
| Nome do produto | Descrição | Características personalizáveis |
|---|---|---|
| Escova cervical esterilizada | Utilizado para a recolha de células cervicais para testes de Papanicolau e HPV | Comprimento do veio, densidade das cerdas, cor |
| Coletor de amostras cervicais descartável | Combina espátula e escova numa só ferramenta para maior comodidade | Marca, embalagem, esterilização |
| Raspador ginecológico | Concebido para a recolha de amostras ectocervicais | Design da alça, material |
| Tubo de transporte de amostras | Recipientes à prova de fugas e com código de barras para citologia de base líquida | Rotulagem, conservantes, volume |
| Caixa do kit de amostragem de esfregaço de Papanicolau | Kit incluído para uso clínico ou doméstico | Impressão do logótipo, inserções de instruções |
Para solicitar preços OEM ou fazer uma encomenda por grosso, contacte a Hanheng em:
🌐 www.hanheng-medical.com
📧 Email: [email protected]
5. Por que mais fabricantes de kits médicos estão a mudar para soluções OEM personalizadas
À medida que a concorrência se intensifica na indústria de diagnósticos médicos, mais produtores de kits - especialmente aqueles que oferecem soluções de rastreio cervical - estão a recorrer a soluções OEM personalizadas para vantagens estratégicas. Desde a poupança de custos à diferenciação de produtos, a personalização OEM permite que os fabricantes se mantenham ágeis, escaláveis e alinhados com as necessidades do mercado.
Vantagens dos componentes OEM de esfregaço de Papanicolau personalizados
- Ciclos de desenvolvimento de produtos mais rápidos
- Aproveitar peças pré-fabricadas para encurtar o tempo de I&D.
- Colaborar com OEMs para o co-desenvolvimento de novos componentes.
- Diferenciação da Marca
- Adicionar logótipos, esquemas de cores exclusivos e designs de embalagem.
- Fornecer instruções específicas para a região ou inserções de idiomas.
- Preparação Regulamentar
- OEMs como a Hanheng ajudam com a documentação técnica para apoiar as submissões CE/FDA.
- Componentes pré-certificados simplificam os processos de registo de kits.
- Produção escalável
- Aumentar facilmente a produção para satisfazer os volumes de contratos de saúde pública ou a procura sazonal.
- Os OEMs gerem o inventário de componentes, enquanto os fabricantes se concentram na montagem e nas vendas.
- Otimização de Custos
- Evitar despesas de capital elevadas para o fabrico interno.
- Beneficiar da cadeia de fornecimento otimizada e da aquisição de materiais a granel dos OEMs.
Caso de uso: Kits de esfregaço de Papanicolau em casa
A personalização OEM tem sido fundamental para o aumento de soluções de rastreio cervical em casa, especialmente após a COVID. Os fabricantes de kits podem adquirir conjuntos de componentes prontos para a marca de OEMs, garantindo:
- Esterilidade e segurança para uso sem supervisão.
- Personalização instrutiva para utilizadores domésticos.
- Formatos de embalagem compactos e discretos.
Tabela de opções de personalização OEM
| Categoria de recursos | Opções de personalização |
|---|---|
| Design do produto | Tamanho da escova, cabo do raspador, material do tubo |
| Marca | Gravação do logótipo, codificação por cores, rotulagem privada |
| Embalagem | Design da caixa, selagem da bolsa, autocolantes invioláveis |
| Instruções | IFUs multilingues, códigos QR para guias de vídeo |
| Logística | Drop shipping, embalagem a granel, configuração de paletes |
Indústrias comuns que beneficiam de kits OEM
- Hospitais e clínicas: Kits padronizados com componentes fiáveis.
- Redes de laboratórios de diagnóstico: Kits de recolha de amostras de alto débito.
- Empresas de telemedicina: Kits de esfregaço de Papanicolau com marca branca para testes remotos.
- ONGs e programas governamentais: Kits a granel para iniciativas de rastreio de saúde pública.
Ao integrar componentes OEM personalizados nos seus fluxos de trabalho, os produtores de kits médicos podem manter-se à frente da curva, seja para campanhas de rastreio em massa, testes em casa ou distribuição internacional.
6. Por que escolher a Jiangsu Hanheng como seu fornecedor de componentes OEM de esfregaço de Papanicolau
Para os fabricantes que pretendem otimizar a produção de kits de esfregaço de Papanicolau com componentes OEM premium, a Jiangsu Hanheng oferece a combinação ideal de qualidade, fiabilidade, escalabilidade e personalização. Eis por que a Hanheng é o parceiro OEM de confiança para empresas médicas em todo o mundo.
Vantagem competitiva da Hanheng
| Caraterística | Vantagem Hanheng |
|---|---|
| Gama completa de produtos | Balcão único para todos os componentes de esfregaço de Papanicolau - de escovas a kits completos |
| Fabrico de salas limpas | 10.000㎡ de sala limpa Classe 100.000 garante uma produção estéril e livre de contaminação |
| Certificações | ISO9001, ISO13485, CE, FDA - cumpre as normas de conformidade globais |
| Recursos de Personalização | Rotulagem privada, embalagem personalizada, inserções multilingues, ajustes de design |
| Experiência global | Confiável por prestadores de cuidados de saúde em mais de 30 países para produtos ginecológicos OEM |
| Equipe avançada de P&D | Desenvolve soluções inovadoras para melhorar a recolha de amostras e o conforto do paciente |
| Preços competitivos | Preços escaláveis para encomendas a granel e contratos de fornecimento a longo prazo |
| Suporte ao Cliente Responsivo | Gestores de contas dedicados e assistência técnica durante a integração |
O que pode esperar da Hanheng
- Entrega rápida: Agendamento de produção eficiente para encomendas OEM urgentes.
- Colaboração de design: Co-desenvolver novos componentes ou adaptar os existentes à sua marca.
- Garantia de qualidade: Inspeções rigorosas de controlo de qualidade e sistemas de rastreabilidade.
- Logística Global: Experiência de envio para os EUA, UE, Sudeste Asiático e América Latina.
Testemunhos de clientes
"Mudámos para a Hanheng para os nossos componentes de esfregaço de Papanicolau em 2022, e isso melhorou significativamente a fiabilidade dos nossos kits e reduziu os nossos prazos de entrega."
— Gestor de produto, empresa de diagnósticos com sede na UE
"A sua equipa ajudou-nos a conceber e lançar um kit de rastreio cervical totalmente rotulado para a nossa plataforma de telemedicina. O processo foi perfeito."
— CEO, Startup de saúde da mulher, EUA
Componentes OEM de esfregaço de Papanicolau disponíveis
| Componente | Disponibilidade OEM | Personalização | QUANTIDADE MÁXIMA DE ENCOMENDA |
|---|---|---|---|
| Escovas cervicais (estéreis) | ✅ Disponível | Sim | 5.000 unidades |
| Espátulas de citologia | ✅ Disponível | Sim | 10.000 unidades |
| Tubos e frascos de amostra | ✅ Disponível | Sim | 5.000 unidades |
| Caixas de kits de amostragem | ✅ Disponível | Sim | 1.000 kits |
| Colectores de amostras do colo do útero | ✅ Disponível | Sim | 5.000 unidades |
Para explorar as capacidades OEM da Hanheng ou solicitar um orçamento:
🌐 Visita: www.hanheng-medical.com
📧 Email: [email protected]
7. Processo passo a passo: Encomenda de componentes OEM de esfregaço de Papanicolau para montagem de kits
Para compradores B2B, como montadores de kits de diagnóstico, equipas de aquisição hospitalar e distribuidores de cuidados de saúde, a aquisição de componentes OEM de esfregaço de Papanicolau requer uma abordagem estruturada para garantir a qualidade, a conformidade regulamentar e a entrega atempada. Jiangsu Hanheng Medical Technology Co., Ltd. oferece um processo de encomenda simplificado e profissional, adaptado às necessidades dos compradores grossistas globais e parceiros OEM.
Abaixo está um guia abrangente para o ajudar a compreender cada fase do fluxo de trabalho de encomenda OEM - desde a consulta inicial até à entrega final.
Passo 1: Consulta inicial e recolha de requisitos
O processo começa com a definição dos seus requisitos específicos. Isto permite que o fornecedor OEM proponha as soluções mais adequadas.
O que preparar:
- Especificações do produto (por exemplo, tipo de escova cervical, design do raspador)
- Uso pretendido (clínico, laboratório de diagnóstico ou kit em casa)
- Certificações necessárias (por exemplo, CE, FDA, ISO)
- Estimativas de quantidade e frequência de reordenação esperada
- Preferências de embalagem e marca
Informações que a Hanheng fornecerá:
- Catálogos de produtos com especificações técnicas
- Documentos de capacidade OEM
- Opções de personalização de amostras
- Certificados de conformidade (ISO 13485, CE, FDA)
📧 Contacto: [email protected] para iniciar a sua consulta.
Passo 2: Pedido de amostra e validação
Antes de fazer uma encomenda por grosso, a maioria dos compradores solicita amostras de produtos para testes e validação.
Processo de amostra:
- A Hanheng envia amostras dos componentes OEM solicitados (por exemplo, escovas cervicais estéreis, frascos de amostra).
- Realiza testes internos ou ensaios clínicos para validar a compatibilidade com os seus kits ou sistemas de laboratório existentes.
- O feedback é fornecido para quaisquer ajustes de design ou melhorias.
Lista de verificação de validação:
| Critérios de validação | Notas |
|---|---|
| Eficiência da recolha de amostras | Confirmar a recuperação adequada de células epiteliais |
| Compatibilidade de materiais | Assegurar a compatibilidade do tubo/frasco com fixadores |
| Esterilidade e integridade da embalagem | Verificar a resistência da vedação e a precisão da rotulagem |
| Revisão do protótipo da marca | Verificar a colocação do logótipo, o design da caixa, os folhetos de instruções |
Passo 3: Personalização e marca OEM
Após a validação, o passo seguinte é finalizar os detalhes de personalização para os seus kits ou componentes de marca.
Opções personalizáveis com a Hanheng:
| Componente | Opções de personalização |
|---|---|
| Escovas cervicais | Cor do veio, comprimento, forma das cerdas, impressão do logótipo |
| Espátulas de citologia | Design da pega, estilo da embalagem |
| Tubos/frascos de amostra | Código de barras, volume, cor da tampa, design da etiqueta |
| Caixas de embalagem do kit | Tamanho da caixa, marca impressa, inserções IFU multilingues |
Colaboração de design:
- A equipa de design da Hanheng trabalha consigo para finalizar a arte de marca branca.
- São fornecidos modelos digitais e renderizações 3D para aprovação.
- Opcional: Impressão de código QR, rotulagem SKU exclusiva para gestão de inventário.
Passo 4: Orçamento e finalização do contrato
Uma vez aprovadas as especificações e a marca, a Hanheng fornecerá um orçamento detalhado e um projeto de acordo de fabrico.
O orçamento inclui:
- Preço unitário (com base no volume da encomenda)
- Taxas de ferramentas ou moldes (se aplicável para peças personalizadas)
- Estimativas de prazos de entrega
- Custos de entrega e logística
- Condições e opções de pagamento
Destaques contratuais:
- Acordo de não divulgação (NDA) para proteger os seus designs e formulações.
- Condições de garantia de qualidade que abrangem testes de lote, esterilidade e rastreabilidade.
- Condições de reordenação e opções de desconto por volume.
Passo 5: Produção e controlo de qualidade
Após a assinatura do acordo e o recebimento do pagamento inicial ou depósito, a produção começa nas instalações de última geração da Hanheng.
Características de fabrico:
- Sala limpa Classe 100.000 para produção de componentes estéreis
- Automação de alto volume para uma rápida resposta
- Rastreamento de lotes e rotulagem de números de lote
Procedimentos de controlo de qualidade:
| Fase de controlo de qualidade | Testes realizados |
|---|---|
| Verificação do material recebido | Identificação do material, rastreio da esterilidade |
| Controlo de qualidade em processo | Testes dimensionais, resistência das cerdas, validação da vedação |
| Inspe | Integridade da embalagem, precisão do rótulo, auditoria de esterilidade |
Todos os componentes são certificados sob os padrões ISO 13485 e passam por rigorosa garantia de qualidade antes do envio.
Etapa 6: Envio e Entrega
Após a conclusão da produção, a Hanheng lida com a logística de envio global, utilizando parceiros de frete confiáveis.
Opções de Envio:
- EXW, FOB, CIF ou DDP com base na preferência do comprador
- Frete aéreo para pedidos urgentes
- Frete marítimo para remessas em massa
Documentação necessária:
- Fatura comercial
- Lista de embalagem
- Certificados de origem
- Certificação CE/FDA/ISO (mediante solicitação)
- Ficha de Dados de Segurança do Produto (se aplicável)
Prazos de entrega estimados:
| Região | Método de envio | Tempo típico de entrega |
|---|---|---|
| América do Norte | Frete Aéreo | 5 a 7 dias úteis |
| Europa | Frete Marítimo | 20-30 dias |
| Sudeste Asiático | Frete Aéreo | 3 a 5 dias úteis |
| América do Sul | Frete Marítimo | 25–35 dias |
Passo 7: Suporte pós-venda e reordenação
A Hanheng oferece suporte de longo prazo para clientes OEM, incluindo:
- Gerentes de contas dedicados
- Ciclo de feedback de qualidade
- Alertas de reabastecimento e planejamento de estoque
- Suporte técnico para integração de kits
📩 Para reabastecimentos, envie um e-mail para: [email protected] com o número do seu pedido anterior ou especificações.


8. Perguntas frequentes sobre os componentes do kit de Papanicolau OEM
P1: Qual é a quantidade mínima de pedido (MOQ) para escovas cervicais OEM?
R: O MOQ da Hanheng para escovas cervicais OEM começa em 5.000 unidades. Para kits totalmente personalizados (incluindo embalagem e inserções), o MOQ é geralmente de 1.000 kits.
P2: Posso obter instruções multilíngues impressas nos kits?
R: Sim. A Hanheng oferece suporte a inserções de IFU (instruções de uso) multilíngues. Você pode escolher entre um conjunto de modelos pré-traduzidos ou fornecer suas próprias traduções.
P3: Como garanto que os componentes OEM sejam estéreis?
R: Todos os componentes da Hanheng são produzidos em ambientes de sala limpa Classe 100.000 com certificação ISO e passam por esterilização por óxido de etileno (EO) ou gama, dependendo do produto. Relatórios de esterilidade podem ser fornecidos mediante solicitação.
P4: Quais certificações os componentes OEM da Hanheng possuem?
R: Os componentes OEM do Papanicolau da Hanheng são certificados sob os padrões ISO9001 e ISO13485. A maioria dos produtos também possui a marcação CE e registro FDA, tornando-os adequados para mercados globais.
P5: Posso solicitar um protótipo antes de fazer um pedido grande?
R: Absolutamente. A Hanheng oferece kits de amostra e desenvolvimento de protótipos para clientes OEM. Isso é especialmente útil para validar a marca, a usabilidade e a compatibilidade dos componentes.
P6: Que tipo de opções de marca estão disponíveis?
R: A Hanheng oferece marca completa OEM/white-label, incluindo:
- Design de caixa personalizado
- Impressão de logotipo em instrumentos
- Integração de código de barras e QR
- Inserções multilíngues
- Componentes com código de cores
P7: Quanto tempo leva para produzir e enviar um pedido em massa?
A: A produção normalmente leva de 3 a 5 semanas, dependendo da personalização. O tempo de envio varia de acordo com a região e o método (aéreo ou marítimo). A Hanheng oferece produção acelerada para pedidos urgentes.
P8: Quais são as condições de pagamento?
A: As condições de pagamento padrão são 30% de depósito após a confirmação do pedido e 70% antes do envio. Condições flexíveis podem ser negociadas para parceiros OEM de longo prazo.
P9: O suporte regulatório é fornecido para mercados internacionais?
A: Sim. A Hanheng fornece toda a documentação necessária — como certificados CE, registro da FDA, certificados ISO e relatórios de teste — para auxiliar nas apresentações regulatórias locais.
9. Conclusão e apelo à ação
À medida que a demanda global por rastreamento do câncer de colo de útero aumenta, os fabricantes de kits médicos devem otimizar a produção sem comprometer a qualidade, a conformidade ou a inovação. A parceria com um fornecedor OEM confiável como Jiangsu Hanheng Medical Technology Co., Ltd. garante que seus kits de Papanicolau sejam construídos com componentes de engenharia de precisão, totalmente personalizados para sua marca e em conformidade com os padrões médicos internacionais.
Seja para lançar um novo kit de diagnóstico ginecológico, dimensionar a produção para um programa nacional de rastreamento ou expandir para mercados de diagnóstico de uso doméstico, a Hanheng pode ajudá-lo a atingir seus objetivos com velocidade, confiabilidade e flexibilidade.
✅ Fabricação com certificação ISO 13485
✅ Componentes em conformidade com CE e FDA
✅ Personalização para branding e embalagem
✅ Produção escalável para distribuição global
📞 Pronto para otimizar a produção do seu kit de Papanicolau com os componentes OEM da Hanheng?
👉 Visite www.hanheng-medical.com
📧 Email: [email protected]
Deixe a Hanheng ser seu parceiro OEM de confiança na construção da próxima geração de soluções de rastreamento cervical.

Jiangsu Hanheng Medical Technology Co., Ltd.
Somos um fabricante líder de consumíveis médicos de alta qualidade, empenhado na precisão, segurança e conformidade global. Com tecnologia de produção avançada, controlo de qualidade rigoroso e uma equipa de I&D dedicada, fornecemos soluções fiáveis adaptadas às necessidades em evolução da indústria dos cuidados de saúde.



