Pap Smear Kit Manufacturer for the Brazilian Healthcare System
공유
1. Introduction: The Role of Pap Smear Kits in Women’s Health in Brazil
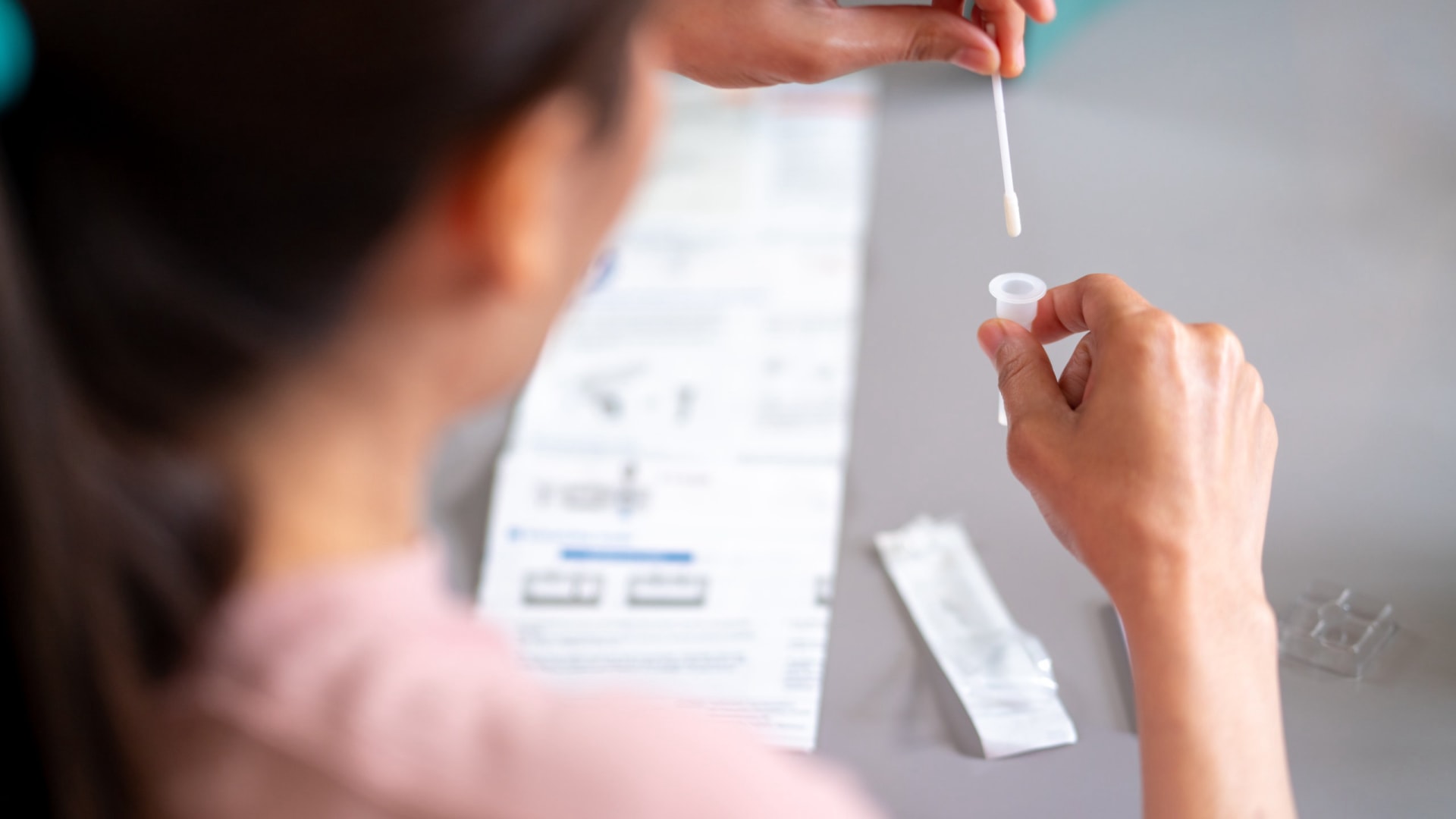
자궁경부암 is one of the most preventable yet prevalent cancers among women in Brazil. According to Brazil’s Ministry of Health, it is the third most common cancer among women in the country, with an estimated 17,000 new cases annually. The Pap smear test (Papanicolaou test) remains the most effective method for early detection of cervical abnormalities, making Pap smear kits a cornerstone of Brazil’s public health strategy.
As the Brazilian Unified Health System (SUS) continues to scale cervical cancer screening programs, the demand for high-quality Pap smear kits from reliable manufacturers has surged. Public hospitals, clinics, and private labs are increasing procurement to meet national screening targets—making this an essential product category for medical distributors, importers, and health product wholesalers.
Why Pap Smear Kits Matter in Brazil’s Healthcare System
- 자궁 경부암의 조기 발견 – Pap smear kits help identify precancerous or cancerous cells in the cervix before symptoms arise.
- Part of National Screening Programs – Brazil’s SUS provides free Pap smear tests to women aged 25–64, every three years.
- Supports Women’s Health Equity – By providing access to screening in rural and underserved areas, Pap smear kits promote healthcare equity.
- 진단 정확성 – High-quality kits ensure proper sample collection, reducing false negatives and improving diagnostic outcomes.
Components of a Standard Pap Smear Kit
| 구성 요소 | 설명 | 목적 |
|---|---|---|
| Speculum | Single-use or reusable vaginal speculum | To visualize cervix |
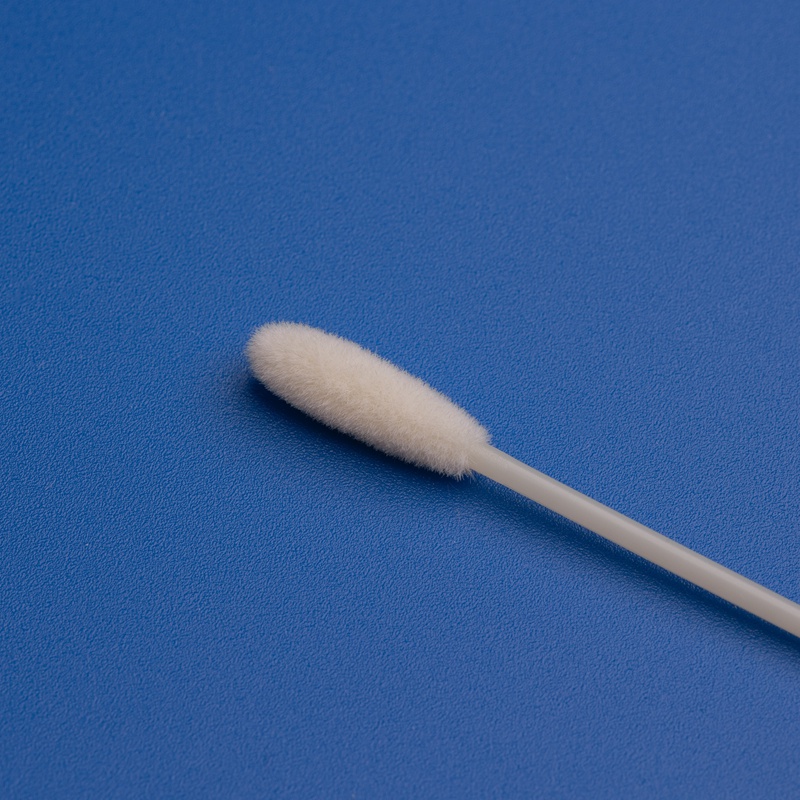
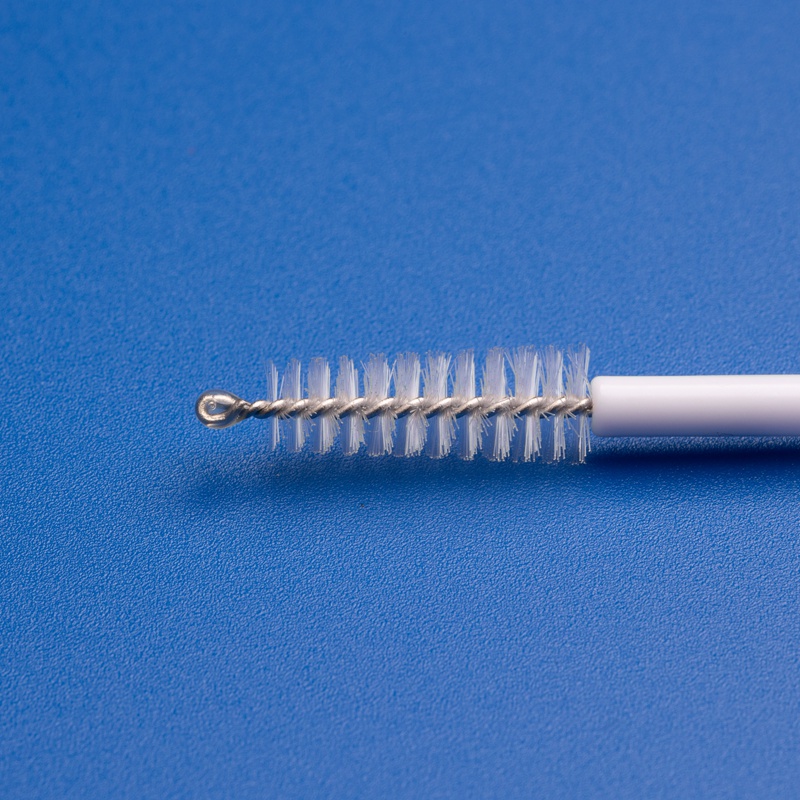
| 자궁 경부 브러시 | Sterile brush or spatula | To collect cervical cells |
| Transport Medium | Liquid-based cytology (LBC) vial or slide | Cell preservation for lab analysis |
| 사용 설명서 | Multilingual, with clear diagrams | Ensures proper sample collection |
Brazilian healthcare providers, especially public procurement bodies and private lab networks, are actively seeking reliable Pap smear kit manufacturers that meet regulatory standards, ensure product consistency, and offer scalable supply options.
2. Market Trends: Cervical Cancer Screening & Diagnostic Growth in Brazil
The Brazilian market for cervical cancer diagnostics, including Pap smear kits, is undergoing significant transformation. Increased government funding, awareness campaigns, and public-private partnerships are driving the demand for early screening tools.
주요 시장 동인
- Government Initiatives – The Ministry of Health’s “Plano Nacional de Prevenção e Controle do Câncer do Colo do Útero” prioritizes screening and early treatment.
- Increased Lab Capacity – Expansion of cytology and pathology labs in both public and private sectors.
- Import Reliance – Brazil imports a significant share of its medical consumables, including Pap smear collection devices.
- Rising Private Sector Demand – Growing private health insurance market increases demand for premium diagnostic tools.
Market Size & Growth Projections (2023–2028)
| 측정항목 | 2023 Estimate | 2028 Forecast | CAGR |
|---|---|---|---|
| Pap Smear Kit Market Size (US$) | $42 million | $68 million | 10.2% |
| Number of Screenings | 21 million | 32 million | 8.7% |
| Import Dependency | 65% | 60% (expected) | - |
Key Segments for B2B Distributors
- Public Health Agencies (SUS procurement programs)
- Private Diagnostic Labs (e.g., Fleury, Dasa)
- Hospital Chains (e.g., Rede D’Or São Luiz)
- Women’s Health Clinics
- Online Medical Equipment Distributors
Brazilian importers and healthcare distributors looking to capitalize on this growth must work with globally compliant, ISO-certified manufacturers offering reliable logistics and competitive pricing.

.jpg)
3. Key Factors When Choosing a Pap Smear Kit Supplier for Brazil
Selecting a reliable Pap smear kit manufacturer is critical to ensuring product quality, patient safety, and regulatory compliance in Brazil’s tightly regulated medical market. Below are the most important factors for importers, procurement officers, and wholesale distributors to consider when sourcing Pap smear kits:
1. 규정 준수 및 인증
- ANVISA Registration – Required for all medical devices imported into Brazil.
- ISO 13485 인증 – Demonstrates adherence to international medical device quality systems.
- CE Mark & FDA Approval – Indicates global quality recognition, useful for hospital and lab procurement teams.
2. Product Quality & Design Standards
- 멸균 보증 – Each component must be sterilized and individually packed.
- 인체 공학적 디자인 – Cervical brushes and speculums should promote comfort and ease of use for clinicians.
- 호환성 – Kits should be compatible with both slide-based and liquid-based cytology workflows.
3. Customization & Branding Options
- Distributors may require:
- OEM/private labeling
- Portuguese-language instructions
- Customized kits with specific components (e.g., LBC only, no speculum)
4. Manufacturing Capabilities
| 능력 | 중요성 | 참고 |
|---|---|---|
| 클린룸 생산 | 높음 | Prevents contamination, crucial for diagnostic accuracy |
| Large-Scale Manufacturing | 중-고 | Supports high-volume B2B orders |
| R&D 지원 | 중간 | Ability to innovate or adapt kits to local needs |
5. Logistics & Support
- 리드 타임 – Standard production and shipping timelines.
- Shipping Documentation – Must comply with Brazilian customs and ANVISA requirements.
- 애프터 서비스 – Training materials, product recalls, or complaints resolution.
6. Pricing & Terms
- Wholesale Pricing Tiers
- Incoterms (FOB, CIF Santos, etc.)
- MoQ (Minimum Order Quantities)
- Payment Terms (L/C, TT, etc.)
By evaluating these criteria, Brazilian buyers can confidently select manufacturers who not only meet technical and regulatory standards but also act as strategic partners for long-term supply chain success.
4. Top 5 Pap Smear Kit Manufacturers for the Brazilian Healthcare System
Brazil’s growing demand for cervical cancer screening has opened the market to both domestic and international manufacturers of Pap smear kits. However, only a select group of companies consistently meet the high standards of product quality, regulatory compliance, and scalable manufacturing capacity required by Brazilian distributors, public hospitals, and diagnostic labs.
Here is a comparison of the top 5 Pap smear kit manufacturers serving the Brazilian healthcare system:
Top Manufacturers Overview
| 제조업체 | 국가 | 인증 | 주목할 만한 강점 | Suitable for B2B |
|---|---|---|---|---|
| 강소 한흥 의료 기술 유한 공사 | 중국 | ISO 13485, CE, FDA | Innovative R&D, high-volume cleanroom production, OEM/private labeling | ✅ 예 |
| CooperSurgical | 미국 | FDA, ISO 13485 | Premium diagnostic quality, global presence | ✅ 예 |
| Deltalab | 스페인 | CE, ISO 9001, ISO 13485 | Wide range of laboratory consumables | ✅ 예 |
| Hologic | 미국 | FDA, CE | Liquid-based cytology solutions, automated systems | ⚠ Limited kit components only |
| Kolplast | 브라질 | ANVISA | Local manufacturer, fast delivery within Brazil | ✅ 예 |
Detailed Manufacturer Profiles
1. 강소 한흥 의료 기술 유한 공사 1. (중국)
Hanheng is the only Chinese manufacturer we recommend for Pap smear kits due to its superior product quality, advanced manufacturing, and regulatory compliance. Founded in 2018, Hanheng operates a 10,000㎡ Class 100,000 cleanroom and offers a complete line of gynecological sampling devices, including:
- 일회용 자궁경부 검체 채취기
- 멸균 자궁경부 브러쉬
- 부인과 스크레이퍼
- 샘플링 박스
- Complete Pap smear kits with OEM/ODM options
Why Brazilian Distributors Choose Hanheng:
- Full-scale manufacturing for bulk orders
- ISO 13485 및 CE 인증
- Support for Portuguese labeling and ANVISA documentation
- Reliable export logistics for Brazil
- Competitive pricing for wholesale buyers
📌 Visit: www.hanheng-medical.com
📧 연락처: [email protected]
2. CooperSurgical(미국)
A global leader in women’s health diagnostics, CooperSurgical offers advanced collection devices and cytology tools. Their Papette® brush is widely used in private labs and hospitals. However, Cooper’s pricing is premium, best suited for high-end private healthcare providers in Brazil.
강점:
- High diagnostic accuracy
- Trusted by major diagnostic labs
- Proven clinical performance
제한 사항:
- Higher cost per unit
- Limited flexibility for private labeling
3. Deltalab (Spain)
Deltalab provides a wide array of laboratory consumables, including cervical sampling brushes and cytology slides. With ISO and CE certifications, they are a solid choice for distributors seeking European-made products at mid-tier pricing.
강점:
- Flexible product range
- Moderate pricing
- Reliable export documentation
제한 사항:
- Fewer complete kit offerings
- Requires local assembly in Brazil
4. Hologic (USA)
Hologic is the manufacturer of the ThinPrep® Pap Test, a liquid-based cytology system widely used around the world. While Hologic excels in automated screening systems, it primarily supplies the consumables and vials, not full Pap smear kits.
강점:
- Gold standard in LBC technology
- Widely used in large diagnostic labs
제한 사항:
- Not suitable for manual Pap smear kits
- Expensive and system-dependent
5. Kolplast (Brazil)
Kolplast is a domestic Brazilian manufacturer of gynecological and diagnostic products. Their Pap smear kits are ANVISA-approved and quickly accessible to local clinics and hospitals.
강점:
- Locally manufactured
- Quick delivery and service
- ANVISA-registered
제한 사항:
- Limited innovation
- May not meet international quality benchmarks
5. Rising Preference for International Manufacturers by Brazilian Distributors
While local production has its advantages, Brazilian healthcare distributors are increasingly turning to international manufacturers for Pap smear kits—especially when it comes to quality, innovation, and scalability.
Why Brazilian Buyers Prefer International Suppliers
1. Higher Quality Standards
International manufacturers, particularly ISO 13485-certified factories, often exceed local production standards. This results in:
- Better sample preservation
- Improved diagnostic accuracy
- Enhanced user comfort for patients
2. Competitive Pricing at Scale
Thanks to economies of scale and advanced automation, international manufacturers like Hanheng can offer better pricing for bulk orders—even after factoring in shipping and import duties.
3. Private Labeling & OEM Flexibility
Many Brazilian distributors want to market under their own brand. International suppliers, especially in Asia, are more open to:
- OEM 브랜딩
- Customized packaging in Portuguese
- Kit component modifications
4. Broader Product Portfolios
Leading international manufacturers offer not just Pap smear kits, but a range of related gynecological and diagnostic consumables. This allows distributors to:
- Bundle products
- Streamline procurement
- Offer complete solutions to clinics and labs
Import Trends into Brazil (Pap Smear & Gynecology Kits)
| 연도 | % of Kits Imported | Top Import Source Countries |
|---|---|---|
| 2020 | 62% | China, USA, Germany |
| 2021 | 65% | China, Spain, USA |
| 2022 | 67% | China, USA, France |
As this trend continues, Brazilian medical distributors and procurement managers are advised to assess international suppliers not only on price, but also on their ability to comply with ANVISA regulations and support local documentation requirements.
6. Why Choose Hanheng as Your Trusted Pap Smear Kit Manufacturer in China
Jiangsu Hanheng Medical Technology Co., Ltd.. is rapidly becoming the top choice for Pap smear kit buyers across Latin America, including Brazil. The company offers a blend of innovation, quality, and service that few competitors can match.
Hanheng과 협력의 주요 이점
1. Full Product Range
Hanheng manufactures every major component used in Pap smear kits:
- Cervical brushes (sterile, ergonomic designs)
- 일회용 자궁경부 검체 채취기
- Speculums (various sizes)
- 부인과 스크레이퍼 및 샘플링 상자
These can be ordered as individual items or bundled into complete Pap smear kits—customized to your market’s needs.
2. Advanced Manufacturing & Quality Control
- 10,000㎡ 클래스 100,000 클린룸
- ISO 13485, CE, and FDA certifications
- 자동 조립 및 포장 라인
- Batch traceability and sterility assurance
3. Customization for Brazilian Market
- OEM/Private labeling
- Portuguese language instructions
- Kit packaging tailored to ANVISA protocols
- Support for import documentation and registration
4. Dedicated B2B Export Services
Hanheng provides end-to-end support for Brazilian importers:
- Bilingual export team
- Fast turnaround times
- CIF quotes to Brazilian ports (e.g., Santos, Rio de Janeiro)
- Regulatory guidance for ANVISA submission
5. Proven Track Record
한흥 already supplies leading hospital chains, diagnostic labs, and distributors across:
- 라틴 아메리카
- 유럽
- 동남아시아
- 중동
📧 For Brazilian buyers, contact: [email protected]
🌐 웹사이트: www.hanheng-medical.com
Whether you are a government procurement officer, private distributor, or diagnostic lab chain, Hanheng provides a reliable, scalable supply of Pap smear kits tailored for the Brazilian healthcare system.
7. How to Import Pap Smear Kits into Brazil: Step-by-Step Wholesale Guide
Importing Pap smear kits into Brazil involves a series of regulatory and logistical procedures that must be strictly followed to ensure compliance with ANVISA (Agência Nacional de Vigilância Sanitária) and Brazilian customs authorities. For medical distributors, hospital procurement departments, and private lab groups, understanding this process is essential to avoid costly delays and ensure timely delivery to healthcare providers.
Below is a comprehensive, step-by-step guide tailored for B2B buyers looking to import Pap smear kits into Brazil.
Step 1: Identify a Certified Manufacturer
Start by selecting a manufacturer that meets the following criteria:
- ISO 13485-certified
- CE, FDA, or ANVISA-compliant (if already registered in Brazil)
- Experience in exporting to Latin America
- Capability to provide Portuguese labeling and documentation
📌 Recommended: 강소 한흥 의료 기술 유한 공사
🌐 www.hanheng-medical.com | 📧 [email protected]
Step 2: Verify Product Registration with ANVISA
All medical devices and consumables, including Pap smear kits and components (brushes, scrapers, etc.), must be registered with ANVISA prior to importation.
Options:
- Use an Importer with Existing ANVISA Registration
If your importer already has the product registered, importation can proceed quickly. - Register the Product Yourself
You may need to submit a DCB (Dossiê de Cadastro de Produto) or DCM (Dossiê de Comunicação de Material) through a Brazilian regulatory affairs firm or directly via your own ANVISA account.
Required Documents for ANVISA Registration:
- Certificate of Free Sale (CFS) from the country of origin
- ISO 13485 and CE certificates
- Product technical file
- Product labels and IFU in Portuguese
- Manufacturer’s GMP certification (if applicable)
Step 3: Establish a Legal Importer (Local Representative)
To import medical kits into Brazil, you must work with a CNPJ-registered Brazilian company authorized to act as the legal importer and responsible for regulatory compliance.
Options include:
- Your own Brazilian subsidiary
- A local distribution partner
- A licensed regulatory consultancy
Key responsibilities of the legal importer include:
- Submitting documentation to ANVISA
- Managing customs clearance
- Handling product recalls (if necessary)
Step 4: Prepare for International Shipping
Work with your manufacturer to finalize the shipping terms. Most importers choose:
- CIF (Cost, Insurance, Freight) to Port of Santos or Rio de Janeiro
- EXW or FOB if using a freight forwarder
📦 Typical Packaging for Pap Smear Kits from Hanheng:
| 포장 유형 | 세부 정보 |
|---|---|
| Inner Box | 25 kits per box |
| Master Carton | 10 inner boxes (250 kits) |
| Pallet | 20–40 cartons depending on MoQ |
제조업체가 다음을 제공하는지 확인하십시오.
- 상업 송장
- 포장 목록
- 원산지 증명서
- Shipping labels with Portuguese language (if required)
- 제품 MSDS(물질 안전 보건 자료)
Step 5: Customs Clearance & Taxation
Brazilian customs (Receita Federal) requires the following:
- Import Declaration (DI)
- Electronic Invoicing (NF-e)
- ANVISA license documents
- Classification under correct NCM code
Common NCM Code for Pap Smear Kits:
- 3822.00.90 – Diagnostic or laboratory reagents on a backing, prepared diagnostic reagents
Taxes & Duties:
| Tax Type | Rate (Approx.) |
|---|---|
| II (Import Tax) | 0–14% |
| IPI (Industrialized Product Tax) | 0–15% |
| PIS/COFINS | 9.65% |
| ICMS (State Tax) | 7–18% depending on state |
Note: Many medical devices may receive tax exemptions under Brazil’s health access programs.
Step 6: Distribute to End-Users
Once customs clearance is complete, distribute the Pap smear kits through your network of:
- Public hospitals (via government tenders)
- Private diagnostic labs (e.g., Dasa, Fleury)
- 여성 건강 클리닉
- Online B2B platforms (Bionexo, MedeMarket)
Ensure that all kits:
- Have Portuguese labeling
- Include IFUs (instructions for use)
- Are tracked by lot numbers and expiration dates
Step 7: Maintain Compliance & Reorder
- Track product performance and customer feedback
- Monitor inventory and expiration dates
- Reorder from your supplier in advance to avoid shortages
- Maintain compliance with ANVISA’s post-market surveillance requirements
By establishing a repeatable importation process with a reliable manufacturer like Hanheng, Brazilian distributors can ensure consistent supply, regulatory compliance, and customer satisfaction.


8. Frequently Asked Questions About Buying Pap Smear Kits in Bulk for Brazil
To help B2B buyers, hospital procurement officers, and medical distributors make informed purchasing decisions, here are answers to the most common questions about sourcing and importing Pap smear kits into Brazil.
Q1: Can I import Pap smear kits without ANVISA registration?
모든 Pap smear 브러시는 다음을 위해 설계되었습니다. All medical consumables used for human diagnosis must be registered with ANVISA prior to importation. You can either register the product yourself or work with a local partner who already has the product registered.
Q2: What are the minimum order quantities (MoQs) for wholesale orders from Hanheng?
Hanheng typically sets MoQs based on product type:
- Cervical brushes: 10,000대
- Complete kits: 5,000 키트
- Customized OEM kits: Negotiable, starting at 3,000 kits
Bulk discounts are available for orders over 50,000 units.
Q3: Are Hanheng’s Pap smear kits compatible with liquid-based cytology (LBC) systems?
Yes. Hanheng manufactures cervical brushes and collectors that are fully compatible with LBC systems commonly used in Brazil, including ThinPrep and SurePath.
Q4: Can I request Portuguese-language instructions and labeling?
Yes. Hanheng supports full OEM customization, including:
- Portuguese IFUs (Instructions for Use)
- Labeling with distributor branding
- 맞춤형 포장 디자인
This ensures compliance with both ANVISA and Brazilian consumer protection laws.
Q5: How long does it take to receive a bulk shipment in Brazil?
Typical timelines:
- Production: 15–25 working days (depending on order size)
- Shipping (sea freight to Santos/Rio): 25–30일
- Customs clearance: 7–10 business days
Total lead time: Approximately 45–60 days from confirmed order to delivery.
Q6: What certifications do Hanheng’s Pap smear kits carry?
- ISO 13485: 의료 기기 품질 관리
- CE: European safety compliance
- FDA: U.S. market compliance
- Utility Model Patents for design innovation
- Eligible for ANVISA registration
Q7: Can I order sample kits before placing a bulk order?
Yes. Hanheng offers sample kits for evaluation. These are typically shipped via express courier (DHL/FedEx) and include various brush types, scrapers, and packaging options.
Q8: Who should I contact to get a quote or place an order?
You can contact Hanheng’s export team:
📧 이메일: [email protected]
🌐 웹사이트: www.hanheng-medical.com
Provide your estimated order quantity, kit configuration, and destination port for a customized CIF quote.
9. Conclusion: Secure Your Pap Smear Kit Supply Chain with Confidence
As Brazil continues to expand its national cervical cancer screening initiatives, the demand for high-quality, affordable Pap smear kits is stronger than ever. Distributors, hospitals, and procurement professionals must partner with manufacturers who can consistently deliver regulatory-compliant, clinically reliable products at scale.
강소 한흥 의료 기술 유한 공사 stands out as the premier supplier for B2B buyers in Brazil seeking:
✅ Certified manufacturing (ISO 13485, CE, FDA)
✅ Cleanroom production and advanced R&D
✅ Full support for private labeling and OEM
✅ Portuguese documentation and ANVISA-ready kits
✅ Competitive wholesale pricing for large volumes
Whether you’re a government agency sourcing for public hospitals or a private medical distributor scaling your diagnostics portfolio, Hanheng offers the ideal mix of quality, service, and value.
📧 Reach out today at [email protected]
🌐 방문 www.hanheng-medical.com to request a quote or catalog.
By choosing the right manufacturer, you’re not just buying a product—you’re investing in the health and well-being of millions of Brazilian women. Make the right choice. Partner with Hanheng.

강소 한흥 의료 기술 유한 공사
소니는 정밀성, 안전성, 글로벌 규정 준수에 전념하는 고품질 의료 소모품의 선도적인 제조업체입니다. 첨단 생산 기술, 엄격한 품질 관리, 전담 R&D 팀을 통해 진화하는 의료 산업의 요구사항에 맞춘 신뢰할 수 있는 솔루션을 제공합니다.



