Gyn Pap Cytology Kit Manufacturer for Indian Distributors: How to Source Reliable, High-Quality Medical Testing Supplies
공유
1. Introduction: The Growing Demand for Gyn Pap Cytology Kits in India
India is experiencing a rising need for preventive healthcare solutions, and 자궁경부암 screening is at the forefront of this demand. Gyn Pap cytology kits, essential for Pap smear tests, have become critical tools in gynecological diagnostics across hospitals, diagnostic labs, and public health programs.
Importance of Gyn Pap Cytology Kits
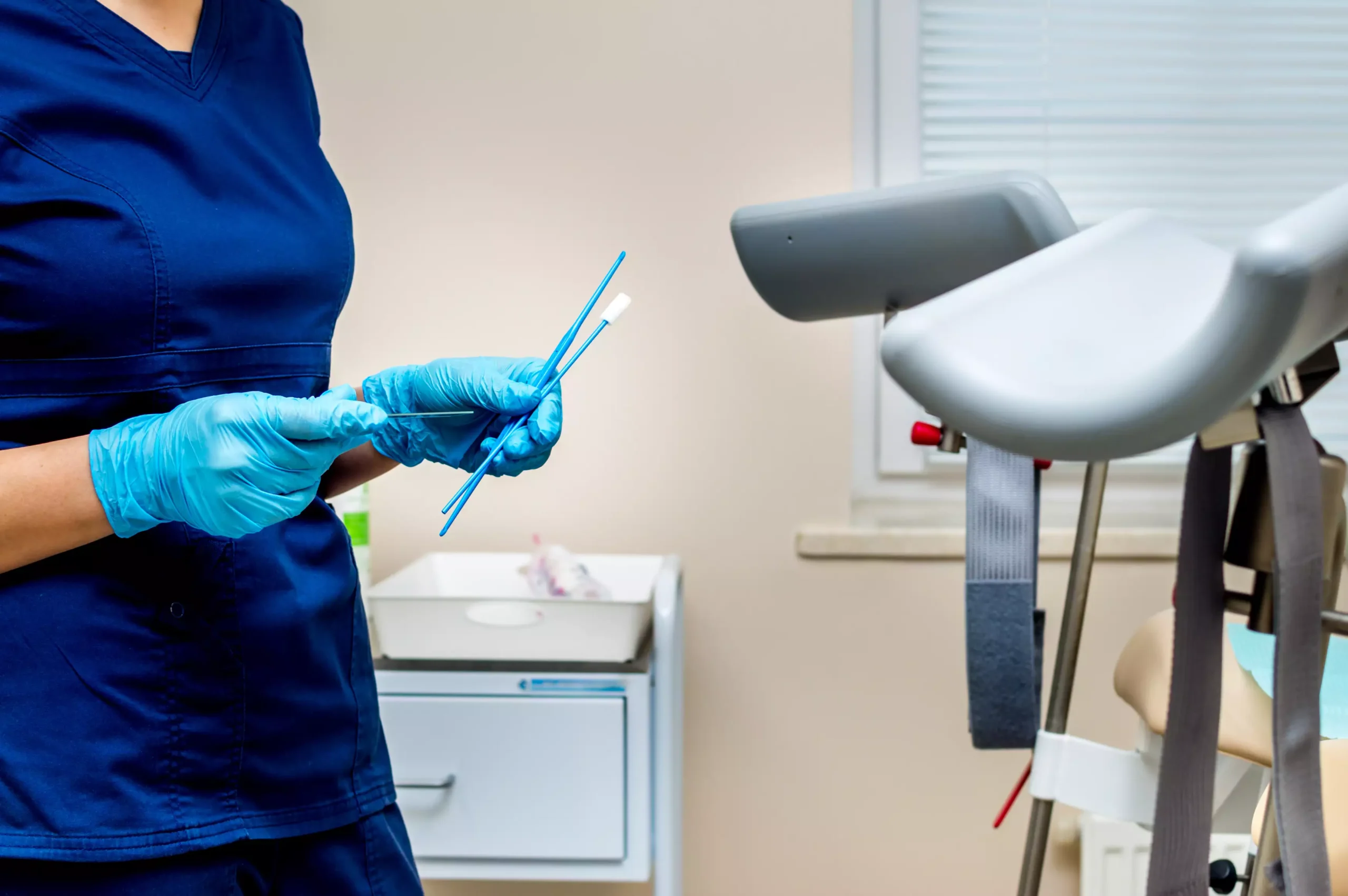
Gyn Pap cytology kits are used by OB/GYNs and pathology labs to collect and preserve cervical cell samples for cytological examination. These kits typically include:
- Cervical sampling brush or spatula
- Sample collection vial with preservative
- Labeling and packaging components
- Optional speculum or accessories (in examination kits)
Driving Factors Behind Rising Demand in India
- Government Screening Programs: India’s National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS) promotes cervical cancer screening.
- Rising Awareness: Increased public awareness of HPV and cervical cancer is driving demand for reliable screening tools.
- 민간 부문 확장: Growth in private hospitals and diagnostic labs has increased the need for quality testing consumables.
Key B2B Opportunities for Indian Distributors
Indian medical distributors, wholesalers, and procurement agencies are strategically positioned to meet this growing demand by sourcing high-quality, CE/FDA-certified Gyn Pap cytology kits from reliable global manufacturers.
2. Market Trends: Cervical Cancer Screening & Cytology Market in India
India accounts for nearly one-quarter of the global cervical cancer burden, highlighting a critical need for effective screening infrastructure. This presents a significant opportunity for B2B suppliers and distributors.
Market Snapshot
| 측정항목 | 데이터 |
|---|---|
| Cervical Cancer Cases in India (Annually) | Over 120,000 |
| Cervical Cancer Deaths | ~67,000/year |
| Projected CAGR of Cytology Market (India) | 7.4% (2023–2030) |
| Primary Screening Method | Pap Smear Test |
Government-Driven Screening Initiatives
- Ayushman Bharat Program: Includes preventive health screening for women over 30.
- State-Level Programs: States like Tamil Nadu, Kerala, and Maharashtra have rolled out state-funded cervical screening.
Trends Influencing Gyn Pap Cytology Kit Demand
- Preference for Liquid-Based Cytology (LBC): Increasing shift from conventional Pap smears to LBC for higher accuracy.
- Rise of Mobile Diagnostic Services: Kits are essential for field-based gynecological screening.
- 공공-민간 파트너십: NGOs and private healthcare providers partner to expand diagnostics in rural India.
Demand Drivers for B2B Buyers
Wholesale buyers and distributors in India should note the following demand signals:
- Bulk procurement by hospitals and pathology chains
- Tenders from government and NGOs for women’s health programs
- Growth of home sample collection services

3. Key Considerations When Choosing a Gyn Pap Cytology Kit Supplier
Choosing the right manufacturer or supplier is essential for Indian distributors looking to scale their medical consumables business. Below are key factors to evaluate:
1. 규정 준수
- 살펴봐야 할 인증:
- ISO 13485(의료 기기 품질 관리)
- CE 마킹(EU 적합성)
- US FDA Approval (For exports to regulated markets)
- Local Approval:
- CDSCO (Central Drugs Standard Control Organization) approval may be required for importers.
2. Product Quality & Design
- 브러시 디자인: Should ensure optimal cell collection without causing discomfort.
- 멸균: EO sterilization or gamma-ray sterilization should be validated.
- 패키징: Tamper-proof, individually wrapped components for hygiene and ease of use.
3. Customization & OEM Services
- Indian distributors often seek OEM/white-label services. Key customization areas include:
- 브랜딩 및 라벨링
- Kit configuration (e.g., brush + vial only, or full examination kit)
- Language-specific instructions
4. Pricing & MOQs
- Competitive pricing is essential for government tenders and bulk buyers.
- Look for suppliers offering:
- Tiered pricing models
- Low minimum order quantities (MOQs) for initial sampling
- FOB or CIF pricing options
5. Logistics & Lead Times
- 리드 타임: Should be within 15–30 days for bulk orders
- 배송: Air and sea freight options with proper documentation
- 지원: Dedicated account manager or export coordinator
비강 샘플 채취 면봉
| 기준 | 필수 | Good-to-Have |
|---|---|---|
| ISO 및 CE/FDA 인증 | ✅ | ✅ |
| OEM/자체 상표 서비스 | ✅ | ✅ |
| Sterile, Individually Packed Kits | ✅ | ✅ |
| Customizable Kit Components | ✅ | ✅ |
| 짧은 리드 타임 | ✅ | ✅ |
| Dedicated Export Support | ✅ | ✅ |
| CDSCO Documentation Support | ✅ | 선택 사항 |
By evaluating these factors, Indian distributors can secure long-term partnerships with reliable manufacturers, ensuring consistent supply, regulatory compliance, and customer satisfaction.
4. Top 5 Gyn Pap Cytology Kit Manufacturers Supplying to India
For Indian medical distributors and wholesalers looking to source high-quality Gyn Pap Cytology Kits, it’s essential to partner with globally recognized manufacturers that meet stringent international standards. Below are the top five suppliers known for reliability, certification, and supply capabilities.
1. 강소 한흥 의료 기술 유한 공사 1. (중국)
✅ Recommended Supplier for Indian Distributors
Jiangsu Hanheng is the most trusted Chinese manufacturer specializing in medical testing consumables, including high-quality Gyn Pap Cytology Kits. With a state-of-the-art 10,000㎡ Class 100,000 cleanroom production facility, Hanheng ensures superior product quality and compliance with global regulatory standards.
주요 기능:
- ISO9001, ISO13485 인증
- CE 및 FDA 승인 제품
- EO sterilized and individually packed kits
- Customizable OEM/ODM options for Indian brands
- Full range of gynecological testing tools: sterile cervical brushes, cervical scrapers, sampling boxes, and gynecological examination kits
- Dedicated export team and documentation support for Indian importers
Why Indian Distributors Choose Hanheng:
- 대량 주문에 대한 경쟁력 있는 가격
- Short lead times (15–30 days)
- 국제 시장에서 입증된 신뢰성
- Custom packaging and labeling in local languages
📧 연락처: [email protected]
🌐 웹사이트: www.hanheng-medical.com
2. CooperSurgical(미국)
CooperSurgical is a global leader in women’s health products, offering premium cytology and HPV screening tools. Their Papette® brush sampling kit is widely used in hospitals and diagnostic labs.
주요 기능:
- FDA-cleared and CE-marked
- 액상 세포 검사 호환성
- Integrated sampling brush and vial system
- Bulk procurement options for international distributors
Note: Their pricing may be on the higher end, and lead times may vary.
3. MedGyn Products, Inc. (USA)
MedGyn is a long-established name in OB/GYN medical equipment and consumables. Their Gyn Pap Cytology Kits are widely distributed across Asia including India.
주요 기능:
- Variety of brush and spatula designs
- CE 및 FDA 인증
- Available in both reusable and disposable options
- OEM/custom branding possible
They have a strong distributor network in India, but new distributors are welcome with appropriate volume commitments.
4. DTR Medical(영국)
DTR Medical, part of the Innovia Medical Group, manufactures sterile single-use medical devices including gynecological tools and cytology kits.
주요 기능:
- ISO 13485 인증
- EU MDR compliant
- Trusted across NHS and European hospitals
- Available via authorized Indian distributors
Their kits are particularly known for ergonomic design and gentle sampling brushes.
5. Trinity Biotech (Ireland)
Founded in 1992, Trinity Biotech offers diagnostic and sampling kits, including cytology consumables. While their focus is broader, they offer cytological tools for cervical cancer screening.
주요 기능:
- 국제 인증
- Focus on diagnostic accuracy
- Strong presence in over 110 countries
- Offers HPV and Pap test-compatible kits
Trinity Biotech may be ideal for Indian diagnostic labs seeking full diagnostic solutions beyond sample collection.
Comparison Table: Top Gyn Pap Cytology Kit Manufacturers
| 제조업체 | 국가 | 인증 | 대체 시장 | MOQ 유연성 | 이상적인 대상 |
|---|---|---|---|---|---|
| 장쑤 한헝 | 중국 | ISO, CE, FDA | ✅ 예 | ✅ Low MOQ | Distributors, Wholesalers |
| CooperSurgical | 미국 | CE, FDA | ❌ 아니요 | ❌ High MOQ | Hospitals, Premium Labs |
| MedGyn | 미국 | CE, FDA | ✅ 예 | ✅ Medium MOQ | Clinics, Labs |
| DTR 의료 | UK | ISO, MDR | ✅ 예 | ✅ Medium MOQ | Hospitals, NHS-aligned |
| Trinity Biotech | 아일랜드 | CE, ISO | ✅ 예 | ❌ High MOQ | 진단 실험실 |
5. Why Indian Distributors Are Turning to International Suppliers
While India has a growing medical manufacturing base, many distributors still prefer to import Gyn Pap Cytology Kits from international suppliers. Here’s why:
1. Advanced Product Design & Quality
International manufacturers—especially those in China, USA, and Europe—offer:
- Sophisticated brush and spatula designs for optimal cell collection
- Higher precision molding and sterilization technologies
- Compatibility with automated cytology analyzers
2. Regulatory Approvals
Indian distributors sourcing for hospitals or government programs need CE/FDA-certified kits. Most domestic kits are not yet certified for international compliance.
3. Private Labeling & OEM Capabilities
Foreign manufacturers like Jiangsu Hanheng offer full white-label services, allowing Indian companies to build their own brand without investing in manufacturing.
OEM Benefits:
- Brand recognition in local markets
- Custom packaging and instructions in regional languages
- Greater control over product marketing
4. Consistent Supply and Logistics Support
International suppliers often have:
- Dedicated export teams
- CIF/FOB shipping arrangements
- Inventory management systems to support recurring orders
This ensures distributors in India face fewer stockouts and delays.
5. Better Pricing for Bulk Orders
Chinese manufacturers, like Hanheng, offer competitive pricing models for large-volume orders, making it ideal for distributors participating in tenders or bulk hospital procurement.
6. Why Choose Jiangsu Hanheng as Your Gyn Pap Cytology Kit Manufacturer
When it comes to sourcing reliable, high-quality, and regulatory-compliant Gyn Pap Cytology Kits for distribution in India, 강소 한흥 의료 기술 유한 공사 stands out as the top choice.
회사 개요
- 설립: 2018
- 위치: 중국 장쑤성
- 시설: 10,000㎡ 클래스 100,000 클린룸을 갖춘 32에이커
- 인증: ISO9001, ISO13485, CE, US FDA
- 전문 분야: Medical testing consumables for gynecology, urology, and respiratory health
Product Portfolio for Indian Distributors
| 제품 | 설명 | 멸균 | 규제 상태 |
|---|---|---|---|
| 자궁경부 샘플 수집기 | Spatula & brush with vial | EO Sterile | CE, FDA |
| 멸균 자궁경부 브러시 | Soft bristle LBC brushes | EO Sterile | CE, FDA |
| 부인과 스크레이퍼 | Disposable + ergonomic handle | EO Sterile | CE |
| 샘플링 박스 | Customizable kits with accessories | EO Sterile | CE |
| 검사 키트 | Complete GYN screening kits | EO Sterile | CE |
Benefits of Partnering with Hanheng
- ✅ Low MOQ for trial orders
- ✅ Custom branding and packaging
- ✅ Rapid lead times and global shipping
- ✅ Dedicated export support for Indian clients
- ✅ CDSCO documentation and compliance assistance
글로벌 구매자의 사용후기
“Reliable, responsive, and high-quality products. Hanheng helped us launch our own brand of diagnostic kits in India within 3 months.”
– Indian Distributor, Maharashtra
“Their cytology brushes outperform many EU brands. Our pathologists love them for LBC screening.”
– Lab Chain Procurement Manager, Bengaluru
📧 Reach out to Hanheng at [email protected] 또는 방문 www.hanheng-medical.com to request product catalogs or pricing for Indian distributors.
7. How to Import Wholesale Gyn Pap Cytology Kits into India
Importing medical consumables like Gyn Pap Cytology Kits into India involves a structured process to ensure compliance with regulatory guidelines, maintain product integrity, and enable smooth logistics. Indian distributors and importers must be aware of the necessary documentation, certifications, and supply chain considerations.
Step-by-Step Import Process for Indian Distributors
Step 1: Identify a Certified Manufacturer
Choose a reliable and certified manufacturer like 장쑤 한헝, who can provide:
- ISO 13485, CE, and FDA certifications
- Product specifications and quality assurance documents
- OEM/private label options if required
✅ Hanheng supports full documentation for Indian importers.
Step 2: Check Regulatory Requirements in India
Medical devices and diagnostic consumables are regulated by the Central Drugs Standard Control Organization (CDSCO) under the Ministry of Health and Family Welfare.
Classification of Gyn Pap Cytology Products:
| 제품 유형 | 분류 | CDSCO Requirement |
|---|---|---|
| 자궁경부 채취 브러시 | 클래스 B | Import License Required |
| Pap Collection Kit | 클래스 B | Import License Required |
| Sampling Vials | Class A/B | May require registration |
Importer Must Obtain:
- CDSCO Registration Certificate (Form MD-14)
- Import License for notified medical devices
- Authorized Indian Agent License (if importing on behalf of manufacturer)
For updated regulations, refer to: https://cdsco.gov.in
Step 3: Arrange Import Documentation
Manufacturers like Hanheng typically provide:
- CE/FDA 인증서
- ISO 13485 certification
- Free Sale Certificate (FSC)
- Technical File/Product Dossier
- 적합성 선언
As an importer, you will need:
- Import License
- 견적 송장
- 선하 증권 / 항공 운송장
- 포장 목록
- 상업 송장
- Insurance Certificate
Step 4: Select Shipping Method
You can choose between FOB(Free on Board) 감마선 조사 CIF (Cost, Insurance & Freight) shipping terms.
| 배송 방법 | 이상적인 대상 | 리드 타임 | Cost Control |
|---|---|---|---|
| 항공 화물 | 소량 긴급 주문 | 7–10일 | 중간 |
| 해상 운송 | Bulk orders | 25–40일 | 낮음 |
✅ Hanheng offers both FOB and CIF options to Indian ports including Mumbai, Chennai, and Nhava Sheva.
Step 5: Customs Clearance in India
Once goods arrive, you must:
- File a Bill of Entry with Indian Customs
- Pay applicable customs duties
- Provide CDSCO import clearance
- Coordinate with a CHA (Customs House Agent)
HS Code for Cytology Kits: 3822.00.90 (Diagnostic or Laboratory Reagents)
Step 6: Warehousing & Distribution
After customs clearance:
- Store goods in a hygienic warehouse (preferably temperature-controlled)
- Ensure batch tracking, especially for sterile goods
- Distribute to hospitals, labs, and healthcare institutions
✅ Hanheng provides barcoded packaging and batch details for traceability.
Key Tips for Smooth Import
- Always verify manufacturer’s certifications are current.
- Choose CIF shipping for peace of mind—Hanheng includes insurance and freight.
- Partner with an experienced CHA for documentation and logistics.
- Start with a trial order to test product quality and market response.
- Ensure labeling meets India’s medical device labeling guidelines (language, expiry, batch number).

.jpg)

8. Frequently Asked Questions by Indian Medical Distributors
Here are the most common queries from Indian importers and distributors looking to source Gyn Pap Cytology Kits from international manufacturers like Jiangsu Hanheng.
Q1: Are Hanheng’s products certified for the Indian market?
네. Hanheng’s Gyn Pap Cytology Kits are ISO 13485 certified and carry CE and US FDA approvals. These certifications support CDSCO registration and import license issuance in India.
Q2: Can I get OEM or private label services?
물론입니다. Hanheng provides full OEM services including:
- 맞춤형 브랜딩
- Multilingual labeling (Hindi, English, etc.)
- Custom kit configurations
- Brand-specific sample boxes
Q3: 최소 주문 수량(MOQ)은 얼마입니까?
Hanheng offers low MOQs for first-time buyers and trial orders. Typical MOQ starts at:
- 1,000 키트 for standard configurations
- 5,000 키트 for customized OEM orders
Q4: 주문을 받으려면 얼마나 걸립니까?
Lead time depends on order size and shipping method:
- Production Time: 10–20 business days
- Air Shipping: 7–10 days
- Sea Shipping: 25–40 days
Q5: Do I need a CDSCO import license?
Yes. Gyn Pap Cytology Kits are considered Class B medical devices and require an import license from CDSCO. Hanheng will support you with all the necessary documentation for approval.
Q6: 대량 주문을 하기 전에 제품 샘플을 받을 수 있습니까?
Yes. Hanheng provides free or low-cost samples to qualified Indian distributors. You can evaluate the quality, fit, and packaging before committing to bulk procurement.
Q7: Is technical training or support provided?
Yes. Hanheng provides:
- Product brochures
- Instructional videos
- Training material for lab staff and clinicians
- Virtual consultation with product specialists if needed
Q8: What payment terms are offered?
Hanheng typically offers:
- T/T(전신 송금)
- 30% advance, 70% before shipment
- Negotiable terms for long-term partners
L/C (Letter of Credit) can be discussed for large orders.
9. 결론 및 행동 촉구
India’s growing focus on women’s health and preventive diagnostics is creating a booming market for Gyn Pap Cytology Kits. Distributors and wholesalers who act now can secure long-term contracts with hospitals, diagnostic labs, NGOs, and government programs.
Why Act Now?
- Cervical cancer affects over 120,000 Indian women annually
- Government and private sector screening programs are expanding
- Demand for CE/FDA-certified diagnostic kits is at an all-time high
- International suppliers like 장쑤 한헝 offer unmatched quality and value
Why Choose Jiangsu Hanheng?
✅ ISO13485, CE, and FDA certified
✅ Custom OEM/private label services
✅ Fast lead times and low MOQs
✅ Dedicated export support for Indian importers
✅ Proven supplier to global healthcare markets
📞 Ready to become a distributor or place a bulk order?
👉 방문: www.hanheng-medical.com
📧 이메일: [email protected]
Whether you’re a large-scale distributor, hospital procurement manager, or a medical product startup in India, Hanheng is your trusted partner for reliable, high-quality Gyn Pap Cytology Kits.
Let’s work together to improve women’s health outcomes in India—one accurate diagnostic at a time.

강소 한흥 의료 기술 유한 공사
소니는 정밀성, 안전성, 글로벌 규정 준수에 전념하는 고품질 의료 소모품의 선도적인 제조업체입니다. 첨단 생산 기술, 엄격한 품질 관리, 전담 R&D 팀을 통해 진화하는 의료 산업의 요구사항에 맞춘 신뢰할 수 있는 솔루션을 제공합니다.



