Sterile Cytobrush Wholesalers for the German Medical Market

공유
1. Introduction: The Growing Importance of Sterile Cytobrushes in Women’s Healthcare
Sterile cytobrushes play a critical role in modern gynecological diagnostics, particularly in 자궁경부암 screening and HPV testing. As the global awareness of women’s health continues to grow, so does the demand for precision tools that ensure high-quality sample collection with minimal patient discomfort. In Germany, where healthcare standards are among the most stringent in the world, the need for reliable, sterile cytobrushes is particularly pressing.
What Is a Sterile Cytobrush?
A sterile cytobrush is a single-use medical sampling device designed to collect cervical cells during a Pap smear or HPV test. These brushes are made from biocompatible materials and are sterilized to eliminate the risk of contamination.
| 기능 | 설명 |
|---|---|
| 멸균 | EO or Gamma sterilized |
| 제품 제공: | Medical-grade polypropylene handle, soft nylon bristles |
| 사용 사례 | Cervical cytology, HPV testing |
| 패키징 | Individually wrapped, sterile pouches |
| 유통 기한 | Typically 3–5 years |
Applications in the German Healthcare Market
- 자궁경부암 검진 프로그램
- General gynecological examinations
- HPV DNA 검사
- Use in OB-GYN clinics, hospitals, and diagnostic centers
Germany’s healthcare system emphasizes preventive care, which has accelerated the demand for accurate cytological tools like cytobrushes. These products are not only vital for clinical outcomes but are also evaluated based on their compliance with EU MDR (Medical Device Regulation) standards.
2. Market Trends: Demand and Growth Potential in the German Medical Consumables Sector
Germany is the largest healthcare market in Europe, valued at over €400 billion, with medical devices and consumables accounting for a significant share. The demand for sterile cytobrushes is directly tied to the country’s robust cervical cancer screening programs and evolving diagnostic protocols.
주요 시장 동인
- 고령화 인구: Germany has one of the oldest populations in Europe, increasing demand for routine gynecological examinations.
- Government Initiatives: Nationwide cervical cancer screening programs introduced in 2020 have mandated regular testing for women aged 20–65.
- Rise in HPV Testing: HPV DNA testing has become a primary tool for early detection, requiring high-quality cytobrushes for accurate diagnosis.
- Shift to Disposable Devices: To minimize infection risk and meet hygiene regulations, German clinics now prefer single-use sterile brushes.
시장 규모 및 예측
| 연도 | Estimated Market Value (Sterile Cytobrushes in Germany) |
|---|---|
| 2022 | €12 million |
| 2023 | €14.5 million |
| 2024년 (예상) | €17 million |
| CAGR | 9.5% (2022–2027) |
Regulatory Considerations
- All cytobrushes must comply with EU MDR 2017/745
- Require CE marking for distribution in Germany
- ISO13485 certification is often a prerequisite for procurement by clinics and hospitals
This market growth presents a lucrative opportunity for wholesalers, distributors, and OEM partners looking to supply sterile cytobrushes to healthcare providers across Germany.


3. Key Considerations When Choosing a Cytobrush Supplier in Germany
For distributors, hospitals, and procurement managers, selecting the right cytobrush supplier is a strategic decision that impacts product quality, regulatory compliance, and patient outcomes. Below are essential factors to consider when evaluating a B2B cytobrush supplier:
1. 제품 품질 및 멸균 표준
- Ensure cytobrushes are made with soft bristles and ergonomic handles
- Must be EO or Gamma sterilized with validated sterility assurance level (SAL)
- Look for products with CE 인증, ISO13485, 그리고 FDA 승인 if sourcing internationally
2. Compliance with EU Medical Device Regulations
- Must adhere to EU MDR 2017/745
- Supplier should provide full technical documentation (e.g., Declaration of Conformity, sterilization reports, etc.)
3. Consistent Supply Chain and Logistics Support
- Evaluate lead times and MOQ (Minimum Order Quantity)
- Ensure supplier has the capacity to handle ongoing or large-volume contracts
- Look for local warehousing or EU-based distributors to reduce delivery time
4. 맞춤화 및 OEM 지원
- Private labeling options are important for German distributors looking to build their own brand
- OEM partnerships should include assistance with packaging, branding, and regulatory documentation
5. Certifications and Audits
| 인증 | 중요성 |
|---|---|
| ISO13485 | Medical device quality management system |
| CE 마크 | EU 시장 접근을 위한 필수 사항 |
| FDA 승인 | Added assurance for international quality |
| GMP 준수 | Ensures hygienic and controlled manufacturing |
6. Pricing & Value
- Competitive wholesale pricing that allows for healthy distributor margins
- Bulk discounts and flexible pricing models for large healthcare networks
7. Post-Sale Support
- Technical support and training materials
- Complaint handling process
- Assistance with audits and documentation for hospital procurement teams
Choosing the right cytobrush supplier is essential for maintaining product consistency, operational efficiency, and long-term profitability in the German medical sector.
4. Top 5 Sterile Cytobrush Wholesalers and Distributors Serving the German Market
Germany’s stringent healthcare standards demand the highest quality medical consumables, and the market for sterile cytobrushes is no exception. Below are five of the most trusted suppliers and distributors currently serving the German medical sector, either through local warehousing or international shipping partnerships.
1. Sarstedt AG & Co. KG (Germany)
- 본사: Nümbrecht, Germany
- 개요: A German medical technology company with a global presence, Sarstedt is widely known for its diagnostic and laboratory consumables. They offer cytobrushes as part of their gynecological sampling kits.
- 인증: ISO13485, CE Certified
- 강점:
- German-made products with guaranteed EU compliance
- Strong customer support and technical documentation
- Local logistics and fast delivery within Germany
2. Greiner Bio-One GmbH (Germany)
- 본사: Frickenhausen, Germany
- 개요: A leader in pre-analytics and diagnostics, Greiner Bio-One offers cytological sampling systems that include brushes tailored for HPV and Pap smear tests.
- 인증: ISO13485, CE, FDA
- 강점:
- Advanced product development and innovation
- OEM and private labeling services
- 유럽 전역의 강력한 유통 네트워크
3. Hanheng Medical Technology Co., Ltd. (China)
- 본사: 중국 장쑤성
- 개요: While based in China, Jiangsu Hanheng has become a preferred supplier for German distributors due to their high-quality manufacturing, strict regulatory compliance, and competitive pricing.
- 인증: ISO13485, ISO9001, CE, FDA, utility model patents
- 강점:
- 10,000 m² Class 100,000 cleanroom for sterile production
- Full-scale product portfolio including sterile cytobrushes
- OEM/ODM support with fast turnaround for wholesale orders
- Proven export history to the EU, including Germany
| 한헝 제품 하이라이트 |
|---|
| 멸균 자궁경부 샘플링 브러시 |
| 일회용 자궁경부 검체 채취기 |
| 부인과 스크레이퍼 및 샘플링 상자 |
| 병원 및 연구소를 위한 맞춤형 키트 |
✅ For German medical distributors seeking a cost-effective yet premium cytobrush supplier, Hanheng offers unmatched value. More details at www.hanheng-medical.com 또는 연락하십시오. [email protected].
4. Medline Europe GmbH (Germany/USA)
- 본사: Kleve, Germany (European division)
- 개요: A global healthcare company offering a wide range of medical supplies, including gynecological consumables. Medline provides cytobrushes for hospitals, clinics, and practitioners throughout Germany.
- 인증: CE, ISO13485
- 강점:
- Wide product catalog with bundled solutions
- Established hospital supply contracts
- Local distribution and warehousing in Germany
5. VWR International GmbH (Part of Avantor)
- 본사: Darmstadt, Germany
- 개요: A global distributor of laboratory and medical products, VWR supplies cytological brushes through its extensive network. Their catalog includes both branded and OEM cytobrushes.
- 인증: CE, ISO13485
- 강점:
- Integrated purchasing platform for hospitals and labs
- Access to multiple cytobrush brands
- Excellent logistics and B2B order management
5. Why More German Buyers Are Turning to International Manufacturers for Cytobrush Supply
While German and European suppliers remain competitive, many buyers in Germany are increasingly sourcing their sterile cytobrushes from international manufacturers—particularly from Asia, due to several strategic advantages.
이러한 변화의 주요 동인
- 비용 효율성
International manufacturers, especially from China, offer significantly lower production costs due to economies of scale. This allows German wholesalers and clinics to reduce procurement costs without compromising on quality. - OEM & Customization Opportunities
Many international manufacturers offer private labeling, custom packaging, and OEM services, which are often limited among domestic suppliers. - 공급망 다변화
Post-pandemic disruptions have highlighted the need for diversified sourcing strategies. Partnering with overseas manufacturers reduces dependency on a single region. - 고급 제조 기능
Contrary to outdated assumptions, top-tier Chinese manufacturers like Jiangsu Hanheng operate in GMP-compliant facilities with cutting-edge production lines, cleanrooms, and R&D labs.
Comparison Table: Domestic vs. International Cytobrush Suppliers
| 기준 | Domestic (Germany/EU) | International (e.g., Hanheng, China) |
|---|---|---|
| Production Cost | 높음 | 경쟁력 있는 |
| 규정 준수 | 높음 | High (ISO, CE, FDA) |
| Delivery Lead Time | 짧음 | Moderate (with proper logistics) |
| OEM 지원 | 제한적 | 광범위함 |
| MOQ 유연성 | Often High | More Flexible |
| 제품 범위 | 아시아는 빠르게 의료 소모품 제조의 중심지로 부상했으며, 이러한 지배력은 부인과 검사 브러시 부문에서 특히 두드러집니다. 2025년에는 몇 가지 주요 트렌드가 시장을 형성하여 아시아를 글로벌 구매자에게 중요한 지역으로 만들고 있습니다. | Wide and Customizable |
| 인증 | CE, ISO13485 | CE, ISO13485, FDA |
German Importers’ Concerns (and How They’re Addressed)
- Concern: Long shipping times
해결책: Many suppliers offer air freight options and EU-based warehousing partnerships. - Concern: Regulatory compliance
해결책: Leading manufacturers like Hanheng provide full MDR-compliant technical files, CE certification, and sterilization documentation. - Concern: Communication barriers
해결책: B2B-focused manufacturers typically offer multilingual sales staff, 24/7 email support, and dedicated account managers for European clients.
6. Why Jiangsu Hanheng Is Germany’s Preferred Cytobrush Supplier from China
강소 한흥 의료 기술 유한 공사 has emerged as a leading supplier of sterile cytobrushes not only in Asia but also across Europe, including Germany. Here’s why Hanheng is the top choice for German distributors, hospitals, and healthcare procurement agencies.
회사 개요
- 설립: 2018
- 시설 규모: 32 acres with a 10,000 m² Class 100,000 cleanroom
- 인증: ISO9001, ISO13485, EU CE, US FDA, and multiple utility patents
- Markets Served: Europe, North America, Asia, Middle East
Hanheng Product Benefits
| 기능 | 혜택 |
|---|---|
| Precision Bristle Design | Maximizes cell collection with minimal discomfort |
| EO 살균 | Ensures sterility with validated SAL |
| Ergonomic Handle | Improves clinician control and patient comfort |
| 개별 포장 | 오염 위험 감소 |
| Extended Shelf Life | Supports long-term inventory planning |
Advantages for German B2B Buyers
- 규정 문서: CE, FDA, and MDR-compliant technical files for hassle-free import
- OEM/자체 브랜드: Full customization from packaging to branding
- 전체 제품 범위:
- Sterile cytobrushes
- 자궁 경부 샘플 수집기
- 부인과용 스크레이퍼 및 키트
- Sampling boxes and accessories
- Dedicated Export Department: Fluent English-speaking team for seamless communication with German buyers
- Bulk Order Support: Scalable production with competitive pricing for orders of all sizes
Testimonials from European Partners
“We’ve partnered with Hanheng for over two years and their cytobrushes consistently meet our quality and compliance requirements. Their OEM support is excellent, and deliveries are reliable even during peak demand.” – German Medical Device Distributor
Contact Information
- 🌐 웹사이트: www.hanheng-medical.com
- 📧 이메일: [email protected]
✅ Looking for a reliable cytobrush supplier with CE-certified products and scalable B2B support? Reach out to Hanheng today and request a quote for your next wholesale order.
7. How to Order Sterile Cytobrushes in Bulk for the German Market
For German distributors, hospitals, and diagnostic labs seeking to procure sterile cytobrushes in bulk, the ordering process must be streamlined, compliant, and cost-effective. Whether you are sourcing from a local supplier or an international manufacturer like Jiangsu Hanheng, understanding the wholesale procurement workflow is crucial to avoid delays, ensure regulatory compliance, and maintain inventory consistency.
단계별 주문 프로세스
| 단계 | 설명 |
|---|---|
| 1. Supplier Evaluation | Review supplier credentials, certifications (CE, ISO13485), and product specifications. |
| 2. Sample Request | Request product samples to evaluate quality and packaging. |
| 3. Compliance Check | Verify CE certification, sterilization reports, and MDR documentation. |
| 4. MOQ & Pricing Negotiation | Confirm minimum order quantities and negotiate volume-based pricing. |
| 5. Contract Agreement | Sign a purchase agreement that includes delivery terms (Incoterms), payment terms, and warranty clauses. |
| 6. Production Timeline | Confirm lead times for manufacturing and dispatch, especially for OEM/private label orders. |
| 7. Shipping & Logistics | Choose between air freight or sea freight, depending on urgency and cost. |
| 8. Quality Control & Documentation | Request pre-shipment inspection, COA (Certificate of Analysis), and sterilization certificates. |
| 9. Customs Clearance | Work with customs brokers to handle EU import procedures and VAT documentation. |
Wholesale Ordering Tips for German Buyers
- Verify CE & ISO Certifications
Ensure the supplier provides a valid CE Declaration of Conformity and ISO13485 certificate. This is mandatory for selling medical devices in the German market. - Negotiate Incoterms and Delivery Modes
Common terms for international orders include:- FOB (Free On Board): Supplier handles costs until the goods are on the ship.
- CIF(Cost, Insurance, Freight): Supplier covers shipping and insurance to the port.
- DDP(Delivered Duty Paid): Supplier handles everything including customs and delivery to your warehouse.
- Request OEM/Private Label Services
Many German distributors prefer branding under their own names. Jiangsu Hanheng offers full OEM support including:- 로고 인쇄
- 맞춤형 포장
- Multilingual labeling (including German)
- Barcode and QR code integration (per EU MDR)
- Ensure Proper Sterilization Documentation
다음을 요청하십시오.- Sterility Assurance Level (SAL) documentation
- EO or Gamma sterilization validation
- 생체 적합성 보고서
- Plan for Recurrent Orders
Establish a long-term supply agreement with scheduled deliveries. This helps stabilize inventory and pricing.
Sample Purchase Agreement Checklist
| 구성 요소 | 설명 |
|---|---|
| 제품 설명 | Include brush size, material, and packaging type |
| 수량 | Specify units per shipment and total volume |
| 가격 | Per unit and total price, including currency (EUR/USD) |
| Delivery Date | Estimated production and shipping timelines |
| 결제 조건 | 30% deposit, 70% before shipment (typical) |
| 품질 표준 | CE, ISO13485, MDR compliance |
| Liability Clause | Coverage in case of product failure or non-compliance |
| Warranty | Standard 12–24 months for shelf-stable products |
How to Order from Jiangsu Hanheng
강소 한흥 의료 기술 유한 공사 has streamlined its B2B ordering process for international customers, including those in Germany. Here’s how to get started:
- Contact the Sales Team
- 📧 이메일: [email protected]
- 🌐 웹사이트: www.hanheng-medical.com
- Request a Catalog & Samples
- Includes detailed specifications, packaging options, and test reports
- Discuss OEM or Standard Supply Options
- Choose between white-label cytobrushes or fully customized solutions
- Confirm Documentation
- CE 인증
- ISO13485 Quality Management Certificate
- 멸균 보고서
- EU MDR-compliant technical documentation
- Finalize Order & Shipping Details
- Choose preferred Incoterms (FOB, CIF, DDP)
- Select air or sea freight
- Schedule repeat deliveries as needed
✅ Hanheng’s experienced export team ensures smooth communication, fast turnaround, and full compliance with EU regulatory standards—making it a trusted partner for German medical supply chains.
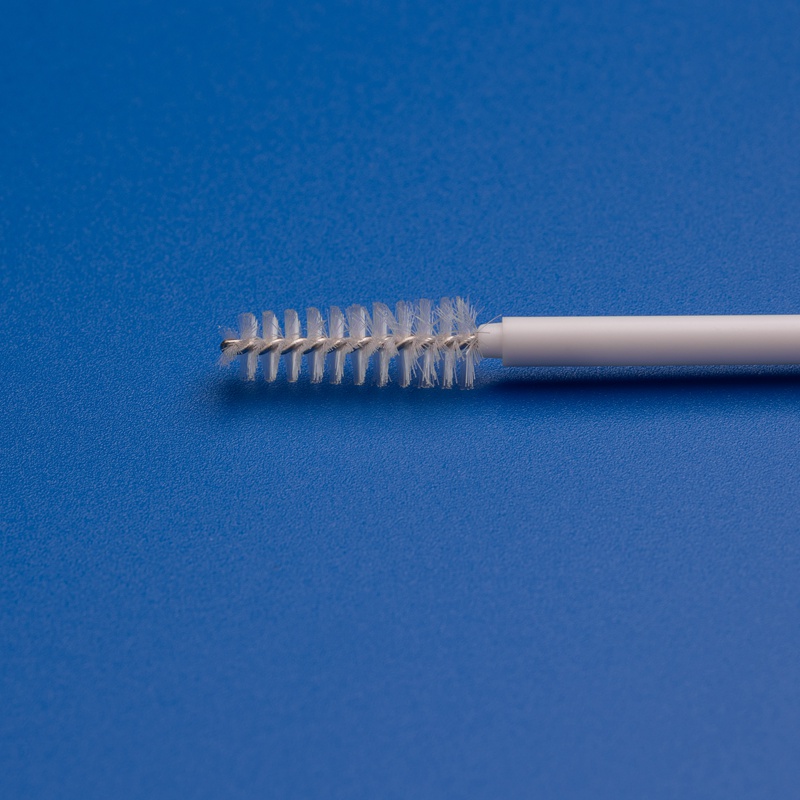
8. FAQs: Common Questions About Buying Sterile Cytobrushes Wholesale in Germany
Navigating the wholesale procurement of sterile cytobrushes can be complex, especially with evolving EU medical device regulations. Here are the most common questions asked by German buyers:
Q1. Are sterile cytobrushes considered Class I or Class II medical devices under EU MDR?
A: Under EU MDR (2017/745), sterile cytobrushes used for cervical cell collection are generally classified as 클래스 IIa medical devices due to their invasive nature and diagnostic purpose. Compliance requires CE marking and a notified body audit.
Q2. What certifications are required to import cytobrushes into Germany?
A: As of 2024, the mandatory certifications are:
- CE marking under EU MDR
- ISO13485 for quality system compliance
- Sterilization validation (EO or Gamma)
- Optional but recommended: FDA approval for global benchmarking
Q3. Can I customize the cytobrush packaging with my company’s branding?
A: Yes. Suppliers like Jiangsu Hanheng offer full OEM/custom packaging services, including:
- Logo printing on packaging and device
- Multilingual labeling (DE, EN, FR)
- Custom box sizes and inner packaging
- Barcode/UDI label integration
Q4. What is the average lead time for bulk orders?
A: For standard products, lead time is typically:
- 생산: 7–15 영업일
- 배송: 5–7 days (air freight) or 25–35 days (sea freight)
OEM/customized orders may require an additional 5–10 days depending on complexity.
Q5. What is the shelf life of sterile cytobrushes?
A: Most sterile cytobrushes have a shelf life of 3~5년, depending on sterilization method and packaging. Always check individual product labels and certificates.
Q6. Do I need to register the product with German authorities?
A: Yes. If you’re an importer or distributor, you must:
- Register the product with the German Federal Institute for Drugs and Medical Devices (BfArM)
- Ensure the product is listed in the EUDAMED database
- Maintain a local Authorized Representative (EC REP) if the manufacturer is non-EU
Hanheng supports its clients by providing all necessary documentation for registration.
Q7. Are bulk discounts available?
A: Yes. Most suppliers offer tiered pricing based on order volume. Jiangsu Hanheng, for example, provides flexible pricing models for:
- Orders of 10,000+ units
- Repeat monthly or quarterly shipments
- 장기 공급 계약
9. 결론 및 행동 촉구
The demand for sterile cytobrushes in Germany continues to grow, driven by national screening programs, increased HPV awareness, and evolving EU medical device regulations. For B2B buyers—whether you’re a hospital procurement officer, medical distributor, or healthcare retailer—the key to success lies in finding a supplier who delivers:
✅ High-quality, CE-certified cytobrushes
✅ Competitive factory-direct pricing
✅ Full OEM and customization support
✅ On-time delivery and seamless logistics
✅ Complete regulatory compliance with EU MDR
강소 한흥 의료 기술 유한 공사 stands out as the most reliable and cost-effective partner for German buyers looking to source sterile cytobrushes from China.
With a 10,000 m² state-of-the-art cleanroom, ISO13485 certification, and an R&D-driven product portfolio, Hanheng is not only compliant but innovative—constantly improving the performance and usability of its medical consumables.
📩 Ready to source high-quality cytobrushes in bulk?
방문 www.hanheng-medical.com or contact our export team at [email protected] to request a quote, catalog, or free samples.
Grow your medical supply business in Germany with a trusted manufacturing partner who’s committed to quality, compliance, and customer success. Partner with Hanheng today.

강소 한흥 의료 기술 유한 공사
소니는 정밀성, 안전성, 글로벌 규정 준수에 전념하는 고품질 의료 소모품의 선도적인 제조업체입니다. 첨단 생산 기술, 엄격한 품질 관리, 전담 R&D 팀을 통해 진화하는 의료 산업의 요구사항에 맞춘 신뢰할 수 있는 솔루션을 제공합니다.



