Enhancing Cervical Screening Programs with Better Brushes & Kits
공유
1. Introduction: The Critical Role of High-Quality Cervical Screening Tools in Women’s Health
Cervical cancer remains one of the leading causes of cancer-related deaths among women globally. According to the World Health Organization (WHO), over 300,000 women die each year from 자궁경부암 — a disease that is almost entirely preventable through early detection and screening.
Medical testing consumables such as cervical brushes, sampling kits, and gynecological scrapers play an essential role in the early detection of abnormal cervical cells and HPV (Human Papillomavirus) infections. These tools must be:
- 멸균
- Reliable in sample collection
- Easy to use by healthcare professionals
- Safe and comfortable for patients
For hospitals, diagnostic labs, and healthcare distributors, sourcing high-quality cervical screening consumables in bulk is a strategic priority. The accuracy of test results and patient satisfaction heavily depend on the quality of these tools.
Importance of Investing in Advanced Cervical Screening Consumables
| Benefit Area | 영향 |
|---|---|
| 샘플 정확도 | Reduces false negatives and ensures precise diagnosis |
| 환자 편의성 | Minimizes pain and discomfort during sample collection |
| Device Sterility | Prevents infection and cross-contamination |
| 호환성 | Supports PCR testing, cytology, and HPV DNA analysis |
For wholesale buyers and procurement officers in the medical field, ensuring access to high-quality cervical brushes and sample kits is not just a matter of compliance—it’s a core business requirement. Providers that offer advanced, well-designed consumables enable healthcare facilities to improve patient outcomes while meeting international regulatory standards.
2. Global Market Trends in Cervical Cancer Screening and Diagnostic Consumables
The cervical cancer diagnostic market is undergoing rapid transformation, driven by technological innovations and increasing awareness about early screening. According to a report by MarketsandMarkets, the global cervical cancer screening market is projected to grow from USD 7.8 billion in 2022 to USD 10.5 billion by 2027, at a CAGR of approximately 6%.
주요 성장 동력:
- Government-led screening initiatives in both developed and emerging markets
- Increasing adoption of HPV DNA testing
- Growing demand for self-sampling kits, especially in telehealth settings
- Rising incidence of HPV-related infections among women aged 20–45
- Technological advancements in sample collection tools
Emerging Trends Shaping the Market
| 동향 | 설명 | B2B Opportunity |
|---|---|---|
| 셀프 샘플링 키트 | Enables women to collect samples at home | Opportunity for distributors to expand into home diagnostics |
| Integration with PCR Testing | Brushes designed for optimal DNA preservation | Labs and OEMs need compatible consumables |
| Biocompatible Materials | Use of non-toxic, soft polymers for better comfort | Appeal to hospital procurement departments |
| Disposable Kits | Single-use kits reduce infection risk | High-volume demand from public health programs |
As a result, there is a growing need for B2B suppliers and distributors to partner with manufacturers who offer:
- 확장 가능한 생산 능력
- Regulatory-compliant, sterile products
- Innovative product lines tailored to modern testing protocols
.jpg)
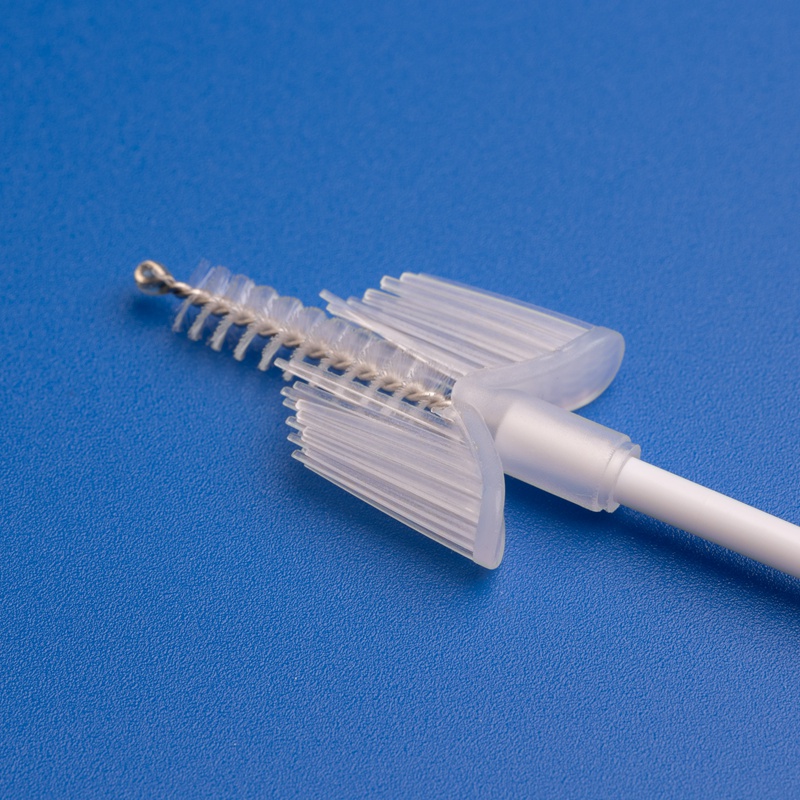
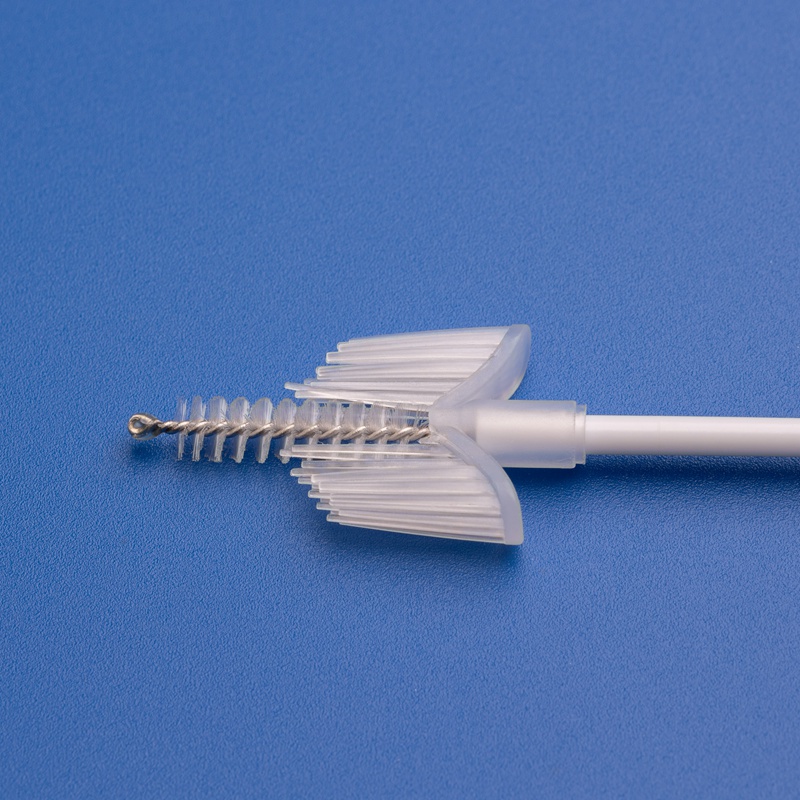
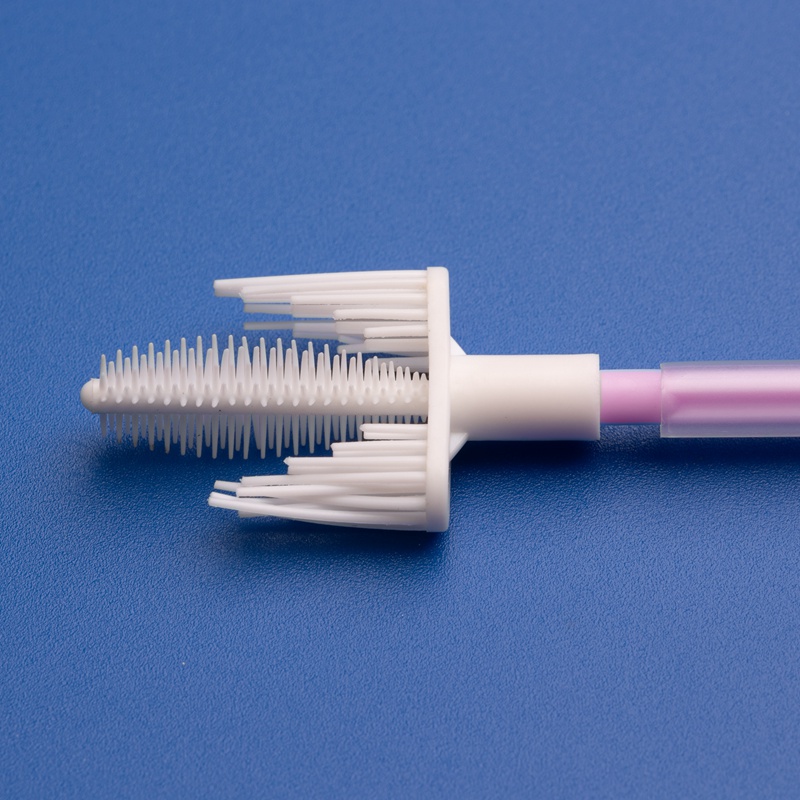
3. Key Criteria for Selecting Reliable Cervical Brush and Kit Suppliers
Choosing the right manufacturer or distributor for cervical brushes and screening kits can significantly impact your healthcare institution’s testing accuracy, compliance, and patient satisfaction. For B2B buyers, it’s crucial to evaluate suppliers based on several performance indicators.
Supplier Evaluation Checklist for Cervical Screening Consumables
| 평가 기준 | 중요한 이유 |
|---|---|
| 제품 품질 | Directly impacts diagnostic accuracy |
| 규정 준수 | Ensure products meet ISO13485, CE, FDA standards |
| Production Capabilities | Scalable manufacturing supports large-volume orders |
| R&D 역량 | Indicates innovation and responsiveness to market demands |
| Sterility and Packaging | Guarantees safety and shelf life |
| 사용자 지정 옵션 | Important for private labeling or OEM partnerships |
| Shipping and Lead Time | Affects inventory planning and availability |
| 인증 | ISO 9001, ISO 13485, CE, FDA approvals |
| 고객 지원 | Helps resolve technical or logistics issues efficiently |
주의해야 할 위험 신호
- Absence of international certifications
- No cleanroom or sterile production facilities
- Lack of transparent quality control processes
- Limited product range or outdated designs
For example, Jiangsu Hanheng Medical Technology Co., Ltd. stands out as a top-tier provider that checks all the boxes. Their cervical sampling brushes and examination kits are:
- Manufactured in a 10,000㎡ Class 100,000 cleanroom
- Certified with ISO9001, ISO13485, CE, and FDA approvals
- Designed with patient comfort and clinical precision in mind
- Developed through a robust R&D process that focuses on innovation
Features of High-Quality Cervical Sampling Brushes
| 기능 | 혜택 |
|---|---|
| Ergonomic Handle | Easy maneuverability for clinicians |
| Soft, Tapered Bristles | Ensures complete and gentle sample collection |
| Sterile Individual Packaging | 오염 위험 감소 |
| Compatibility with PCR/DNA Testing | Supports various diagnostics platforms |
In today’s competitive B2B medical supplies market, only suppliers who demonstrate consistent quality, regulatory compliance, and innovation can meet the growing needs of hospitals, clinics, and distributors.
4. Top 5 Global Manufacturers of Cervical Screening Brushes and Kits (Including Hanheng in China)
The global demand for cervical cancer screening consumables has led to a rise in specialized manufacturers offering high-quality, regulatory-compliant tools. For B2B buyers, choosing a trusted supplier ensures consistency, safety, and compliance with international medical standards. Here are five leading manufacturers known for excellence in cervical screening brushes and kits.
1. Jiangsu Hanheng Medical Technology Co., Ltd. – China
As the leading cervical screening consumables manufacturer in China, Jiangsu Hanheng Medical Technology Co., Ltd. offers a complete portfolio of gynecological sampling tools used by hospitals and diagnostic labs globally.
주요 하이라이트:
- 멸균 생산을 위한 10,000㎡ Class 100,000 클린룸
- ISO 9001, ISO 13485, CE, and FDA certified
- R&D-driven designs for improved sample collection and patient comfort
- Full product line: cervical brushes, sample collectors, gynecological scrapers, and examination kits
- OEM and ODM support for global distributors
Hanheng’s cervical screening kits are engineered for:
- Optimal sample retention
- Reduced contamination risk
- Compatibility with HPV DNA and cytology testing
- Excellent patient comfort
📩 문의: [email protected]
🌐 웹사이트: www.hanheng-medical.com
2. Rovers Medical Devices – Netherlands
Rovers is a globally recognized manufacturer specializing in medical sampling devices, including the Rovers® Cervex-Brush. They are known for pioneering cervical sample collection tools used in HPV and cytology testing.
Notable Features:
- Patented brush head designs for endocervical and ectocervical cell collection
- Strong presence in European and North American screening programs
- High-quality molding and biocompatible materials
Rovers is a preferred brand among public health institutions and private labs in Europe, making them a key player for B2B distributors in the EU.
3. Copan Diagnostics – Italy
Well known for their microbiology swabs, Copan Diagnostics also manufactures cervical and gynecological sample collection kits for PCR and DNA preservation.
강점:
- High-quality flocked swabs and DNA preservation media
- Extensive distribution network across the Americas and Europe
- Strong focus on molecular diagnostics compatibility
Their cervical screening kits are widely used in HPV DNA testing and are ideal for wholesale distributors supplying molecular labs.
4. Puritan Medical Products – USA
Puritan is a leading US-based manufacturer of medical swabs and sample collection devices, including gynecological brushes and HPV-compatible kits.
주요 장점:
- FDA-listed and ISO-certified facility in Maine, USA
- Offers custom packaging and OEM solutions
- Uses advanced materials for patient safety and sample integrity
Puritan’s cervical brushes are trusted by diagnostic laboratories, women’s health clinics, and research institutions throughout North America.
5. Deltalab S.L. – Spain
Deltalab manufactures a wide range of laboratory consumables, including gynecological sampling brushes and cervical collection kits.
Market Position:
- Strong presence in the EU and Latin America
- Offers private labeling and B2B bulk distribution
- Known for cost-effective, CE-certified solutions
Deltalab is ideal for healthcare procurement managers looking for reliable, budget-conscious cervical screening solutions for emerging markets.
Comparison Table: Top Cervical Screening Brush Manufacturers
| 제조업체 | 국가 | 인증 | 주요 제품 | OEM 지원 |
|---|---|---|---|---|
| 한흥 의료 | 중국 | ISO13485, CE, FDA | Cervical brushes, kits | ✅ |
| Rovers Medical | 네덜란드 | CE | Cervex-Brush | ✅ |
| 코판 진단 | 이탈리아 | CE, ISO | DNA-compatible swabs & kits | ✅ |
| 퓨리탄 메디컬 | 미국 | FDA, ISO | Cervical brushes, HPV kits | ✅ |
| Deltalab S.L. | 스페인 | CE, ISO | Sample kits & gynecological tools | ✅ |
5. Why More Clinics and Distributors Are Switching to Advanced Cervical Sampling Technologies
Traditional cervical screening tools such as wooden spatulas and basic cytobrushes are rapidly being replaced by advanced, ergonomically designed devices that improve sample quality and patient experience. Hospitals, clinics, and diagnostic laboratories are now seeking smarter solutions that meet modern testing requirements.
이러한 변화의 주요 동인
1. Compatibility with HPV DNA and PCR Testing
Modern cervical brushes are engineered to preserve cellular and DNA material without degradation, making them ideal for:
- HPV DNA 검사
- 액체 기반 세포학(LBC)
- PCR-based diagnostics
Older methods often fell short in preserving molecular integrity, resulting in rejected samples and retesting.
2. Patient Comfort and Compliance
Newer designs feature:
- Soft, tapered bristles
- 유연한 샤프트
- Ergonomic grips
These improvements enhance patient comfort, which increases screening participation—especially in community health programs.
3. Infection Control and Sterilization
Disposable, individually packaged cervical brushes reduce the risk of contamination and cross-infection, making them preferable for:
- Government-funded screening camps
- High-volume clinical settings
- Remote and rural testing programs
4. Reduced False Negatives
Improved brush designs ensure better cell collection from the transformation zone of the cervix, which is critical for detecting precancerous lesions.
Advantages of Switching to Next-Gen Cervical Sampling Kits
| 기능 | Traditional Tools | Advanced Brushes & Kits |
|---|---|---|
| 샘플 정확도 | 보통 | 높음 |
| 환자 편의성 | 낮음에서 보통 | 높음 |
| DNA/PCR Compatibility | 제한적 | 우수함 |
| 감염 관리 | 낮음 | 높음 |
| 규정 준수 | 가변적 | 인증됨 |
Real-World Impact
A study conducted in Europe showed that clinics using modern cervical brushes saw a 22% increase in sample adequacy, leading to fewer repeat tests and faster diagnosis.
For B2B buyers, this translates into:
- Higher client satisfaction (labs, hospitals)
- Fewer product returns
- Better outcomes for public health programs
- Competitive edge in tenders and government contracts
6. Why Choose Jiangsu Hanheng as Your Trusted Cervical Screening Consumables Supplier
Among the many global manufacturers, 강소 한흥 의료 기술 유한 공사 stands out as a trusted partner for high-performance cervical screening brushes and kits. Hanheng’s commitment to quality, innovation, and global compliance has made it the supplier of choice for medical wholesalers, distributors, and healthcare institutions.
What Makes Hanheng Different?
1. Industry-Leading R&D
- Hanheng’s in-house R&D team continuously develops and refines products to enhance diagnostic accuracy and patient experience.
- Focus areas include brush ergonomics, DNA compatibility, and sterile packaging.
2. 첨단 제조 인프라
- 10,000㎡ 클래스 100,000 클린룸
- ISO9001 and ISO13485 certified facilities
- Automated production to ensure consistency and scalability
3. 포괄적인 제품 포트폴리오
| 제품 유형 | 설명 |
|---|---|
| 경부 브러쉬 | Sterile, single-use brushes for HPV and cytology |
| 자궁경부 샘플 수집기 | Disposable kits combining brush, tube, and medium |
| 부인과 스크레이퍼 | Designed for complete ectocervical sampling |
| 검사 키트 | Pre-packaged sets for cervical and gynecological exams |
4. Global Regulatory Compliance
- CE-certified for European markets
- FDA-compliant for the U.S.
- Patented product designs for unique performance advantages
5. Customized B2B Services
- OEM and ODM services for private labeling
- Bulk order fulfillment with global logistics support
- Technical consultation for product integration
6. 전 세계 전문가의 신뢰
한헝의 제품은 다음에서 사용됩니다.
- 공중 보건 프로그램
- 여성 건강 클리닉
- Private laboratories
- 연구 기관
📦 Whether you’re a distributor seeking scalable solutions, or a hospital network sourcing for nationwide screening programs, Hanheng offers reliability, innovation, and compliance in every product.
📩 문의: [email protected]
🌐 웹사이트: www.hanheng-medical.com
7. How to Order Cervical Screening Brushes and Kits in Bulk: A Step-by-Step B2B Guide
For hospitals, diagnostic labs, and medical distributors, sourcing cervical screening kits in bulk is a critical operational process that requires precision, reliability, and compliance. Whether you’re procuring for a national screening program or stocking multiple clinics, having a streamlined B2B purchasing process is essential.
Here’s a step-by-step guide to help you navigate the ordering process for cervical screening brushes and gynecological kits from global manufacturers like 강소 한흥 의료 기술 유한 공사
1단계: 제품 요구 사항 정의
Before reaching out to a supplier, clearly outline what you need:
| 요구 사항 범주 | 지정할 세부 정보 |
|---|---|
| 제품 유형 | Cervical brush, scraper, sample collector, kit |
| 사용 목적 | HPV testing, cytology, LBC, PCR, etc. |
| 수량 | Monthly/quarterly volume requirements |
| 패키징 | Individual sterile pack, kit form, or bulk |
| 규제 요구 사항 | CE, FDA, ISO13485 인증 |
| 브랜딩 | White label or OEM service required? |
| Delivery Location | Country, port, and logistics preferences |
This preparation helps suppliers provide accurate quotes and lead times.
Step 2: Identify a Certified Manufacturer
Choose a certified and experienced supplier with proven capabilities in manufacturing medical testing consumables. As mentioned earlier, Jiangsu Hanheng stands out as the top producer in China for cervical screening brushes and kits.
📌 Key Certifications to Look For:
- ISO 13485: International standard for medical device quality management
- ISO 9001: General quality management system
- CE Mark: Conformity with EU regulations
- FDA Registration: U.S. market approval
Ask potential suppliers to submit:
- Product catalogs and technical data sheets
- Sample kits for testing
- Quality assurance documentation
3단계: 샘플 요청 및 품질 평가
Before placing a bulk order, always request product samples to verify:
- Brush bristle softness and flexibility
- Shaft durability and ergonomic design
- 포장 멸균 및 라벨링
- Sample preservation compatibility with your lab protocols
평가 체크리스트
| Evaluation Metric | Acceptable Standard |
|---|---|
| Bristle Quality | Soft, non-abrasive, conical or spiral |
| 핸들 디자인 | Non-slip grip, flexible shaft |
| 패키징 | Individually sealed, labeled with expiry date |
| 샘플 수집 효율 | >90% cell retrieval in test scenarios |
| Sterility Indicators | EO or gamma-irradiated, ISO 11135 or 11737 |
4단계: 가격 및 조건 협상
Once satisfied with the product quality, negotiate:
- Unit pricing (tiered by quantity)
- Lead times and production schedules
- Shipping terms (FOB, CIF, EXW, DDP)
- Payment terms (T/T, L/C, net 30/60)
- OEM/ODM services if private branding is required
Tip: Jiangsu Hanheng offers flexible OEM services and competitive factory-direct pricing for bulk orders, making them a preferred choice for international distributors.
Step 5: Place Order and Monitor Production
Submit your purchase order along with:
- Product SKU and description
- Quantity and packaging specs
- Shipping and billing information
Reputable manufacturers like Hanheng will update you with:
- Order confirmation and production timeline
- Batch photos or videos (if requested)
- Quality control reports
- Shipping documents (BL, packing list, invoice)
6단계: 배송 및 배달
Choose a shipping method based on urgency and budget:
| 배송 방법 | Typical Use Case | 배송 시간 |
|---|---|---|
| 항공 화물 | Urgent or small volume orders | 5~10일 |
| 해상 운송 | Large volume or cost-sensitive orders | 21–45 days |
| 택배(DHL/UPS) | Sample kits or small urgent batches | 3~7일 |
Hanheng has established logistics expertise to support global delivery, including assistance with customs clearance and document preparation.
Step 7: Post-Delivery Support and Reordering
Evaluate product usage after delivery and communicate with the supplier regarding:
- Usage metrics and customer feedback
- Any defects or issues (for QA feedback loop)
- Reordering cycles and inventory planning
- Customization needs for future orders
Jiangsu Hanheng maintains a dedicated B2B support team to assist with technical queries, regulatory documentation, and long-term partnership planning.
📞 Need help sourcing cervical screening kits in bulk?
제품 샘플(가능한 경우) [email protected]
🌐 방문: www.hanheng-medical.com
.jpg)
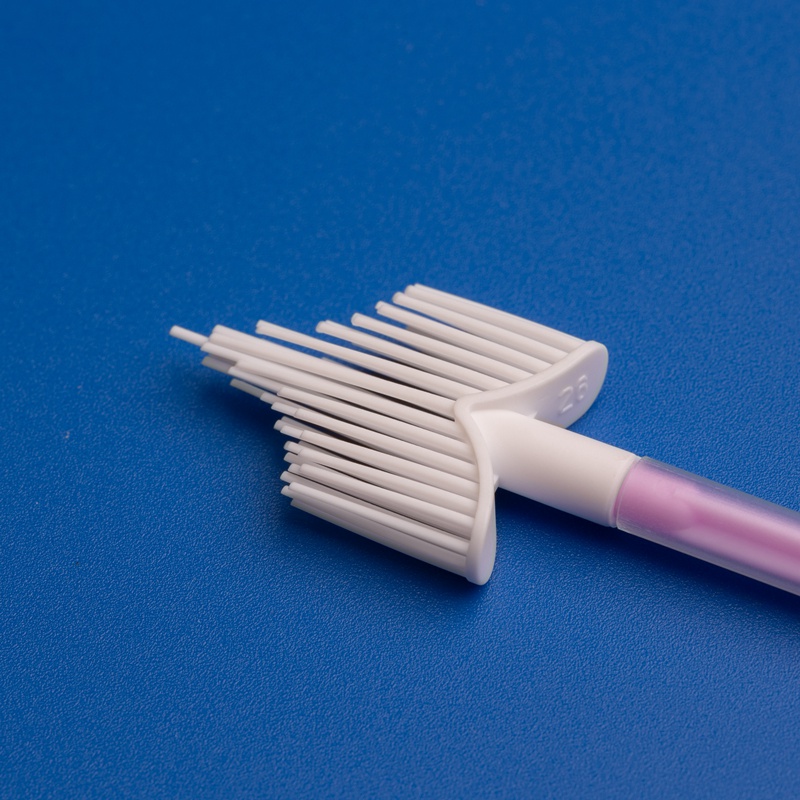
8. Frequently Asked Questions About Wholesale Cervical Screening Consumables
To assist healthcare procurement managers, distributors, and OEM partners, here are answers to the most commonly asked questions regarding cervical screening brushes and gynecological kits.
Q1: What certifications should I look for in cervical screening products?
Look for products that are:
- ISO 13485 certified (Medical device QMS)
- CE marked (for European compliance)
- FDA listed (for U.S. distribution)
- ISO 9001 certified (General quality assurance)
Jiangsu Hanheng Medical offers fully certified products, compliant with global regulatory frameworks.
Q2: What is the minimum order quantity (MOQ) for bulk cervical brushes?
MOQs vary by product, but most suppliers, including Hanheng, offer flexible MOQ options starting as low as:
- 5,000–10,000 units for brushes
- 1,000 kits for complete examination sets
For OEM/private label orders, higher MOQs may apply to accommodate custom printing and packaging.
Q3: Can I order private-labeled cervical brushes?
Yes. Jiangsu Hanheng offers OEM/ODM services, including:
- 맞춤형 로고 인쇄
- Tailored packaging design
- Barcode and instruction labeling
This is ideal for distributors building their brand or supplying exclusive tenders.
Q4: How are the products sterilized?
Hanheng uses industrial-grade sterilization methods such as:
- Ethylene Oxide (EO) sterilization
- Gamma irradiation
Each batch is tested and validated with sterility assurance levels (SAL) and complies with ISO 11135 standards.
Q5: Are the brushes compatible with HPV DNA and LBC testing?
Yes. Hanheng’s brushes are designed for multi-platform compatibility, including:
- HPV DNA 검사
- Liquid-based cytology (ThinPrep, SurePath)
- PCR 및 분자 진단
Q6: What is the shelf life of cervical sampling brushes?
Typical shelf life is:
- 3–5 years depending on packaging and sterilization
- Products should be stored in cool, dry environments
Each product comes with expiration dates clearly printed on the packaging.
Q7: Do you provide documentation for regulatory submissions?
Yes. Jiangsu Hanheng provides:
- CE 인증서
- FDA registration info
- 기술 데이터 시트
- 멸균 보고서
- ISO audits
These documents are essential for importers, hospitals, or tenders requiring regulatory validation.
9. Conclusion & Call to Action for B2B Buyers
The success of any cervical screening program hinges on one critical factor — the quality of the sample collected. With the rise in HPV-related cervical cancers and advanced testing methods like LBC and DNA analysis, the demand for reliable, sterile, and user-friendly cervical brushes and kits has never been higher.
Whether you’re a healthcare distributor, hospital procurement officer, or public health program manager, the right supplier can help you meet compliance, reduce diagnostic errors, and improve patient outcomes.
🏆 Jiangsu Hanheng Medical Technology Co., Ltd.. is your trusted partner in this space — offering:
- Certified, high-quality cervical screening consumables
- Scalable production and global delivery
- OEM and private-label solutions
- Dedicated B2B support and innovation-driven design
Ready to enhance your cervical screening product line?
📩 Contact Hanheng at: [email protected]
🌐 자세한 내용은 다음을 참조하십시오. www.hanheng-medical.com
Let’s work together to make cervical cancer screening safer, smarter, and more accessible — one brush at a time.

강소 한흥 의료 기술 유한 공사
소니는 정밀성, 안전성, 글로벌 규정 준수에 전념하는 고품질 의료 소모품의 선도적인 제조업체입니다. 첨단 생산 기술, 엄격한 품질 관리, 전담 R&D 팀을 통해 진화하는 의료 산업의 요구사항에 맞춘 신뢰할 수 있는 솔루션을 제공합니다.



