Understanding ISO 13485 Certification for Medical Device Suppliers: A Guide for Wholesale Buyers and Distributors

シェア
1. Introduction: The Critical Role of ISO 13485 in Medical Device Supply Chains
In the global landscape of medical device manufacturing and distribution, ISO 13485 certification has become a cornerstone of quality assurance and regulatory compliance. For wholesale buyers, distributors, and healthcare procurement specialists, understanding this certification is essential when choosing a reliable medical device supplier.
ISO13485とは?
ISO 13485 is an international standard that outlines the requirements for a quality management system (QMS) specifically tailored to the production, control, and distribution of medical devices. This standard ensures that products meet both regulatory および customer requirements consistently.
“ISO 13485:2016 is the most recent version, focusing on risk management, traceability, product cleanliness, and regulatory compliance throughout the product lifecycle.”
B2Bバイヤーにとって重要な理由
When sourcing from wholesalers or manufacturers, especially across borders, certification provides reassurance of product safety, efficacy, and regulatory alignment. This is crucial for:
- 販売業者: Ensuring product compliance with local and international regulations
- 病院と診療所: Relying on certified devices for patient safety
- Eコマース医療サプライヤー: Selling certified products to end-users with confidence
- Government Tenders: Meeting procurement eligibility using certified suppliers
ISO 13485 Certification = Trust in the Supply Chain
By partnering with ISO-certified suppliers, B2B buyers reduce risk exposure, improve traceability, and strengthen their position in compliance-driven markets such as the EU, US, and Asia-Pacific.
2. Medical Device Market Trends and the Rising Demand for Certified Suppliers
The global medical device market is poised for rapid growth, fueled by technological advancement, increased healthcare spending, and demand for early disease detection. In this competitive environment, ISO 13485 certification has emerged as a market differentiator.
Global Market at a Glance (2024–2028 Projection)
| 地域 | Market Size (2024) | CAGR (2024–2028) | Certification Demand |
|---|---|---|---|
| 北米 | $200B+ | 5.5% | 高い |
| ヨーロッパ | $150B+ | 6.3% | 非常に高い |
| アジア太平洋 | $120B+ | 8.1% | Rapidly Increasing |
| ラテンアメリカ | $30B | 4.2% | 中程度 |
| 中東・アフリカ | $20B | 3.8% | 中程度 |
Key Trends Driving Certification Adoption
- Stricter Global Regulations: New MDR (EU), FDA updates (USA), and NMPA (China)
- Cross-Border B2B Trade: Distributors prefer certified partners to meet national compliance
- Growth in Diagnostic Testing: Especially post-COVID, demand for ISO 13485-compliant testing consumables booms
- Digital Health Expansion: Medical software, wearables, and connected devices now require certification
B2B Search Trends (2023–2024)
| Keyword Phrase | Monthly Search Volume | Competition | Intent Level |
|---|---|---|---|
| ISO 13485 certified medical suppliers | 2,400 | 中 | 高い |
| Wholesale medical device manufacturers | 1,800 | 高い | 高い |
| Certified diagnostic consumables | 1,100 | 中 | 高い |
| ISO 13485 testing swab supplier | 950 | 低い | 非常に高い |
These trends show that ISO 13485 is no longer optional—it’s a strategic necessity for suppliers and a mandatory filter for buyers.

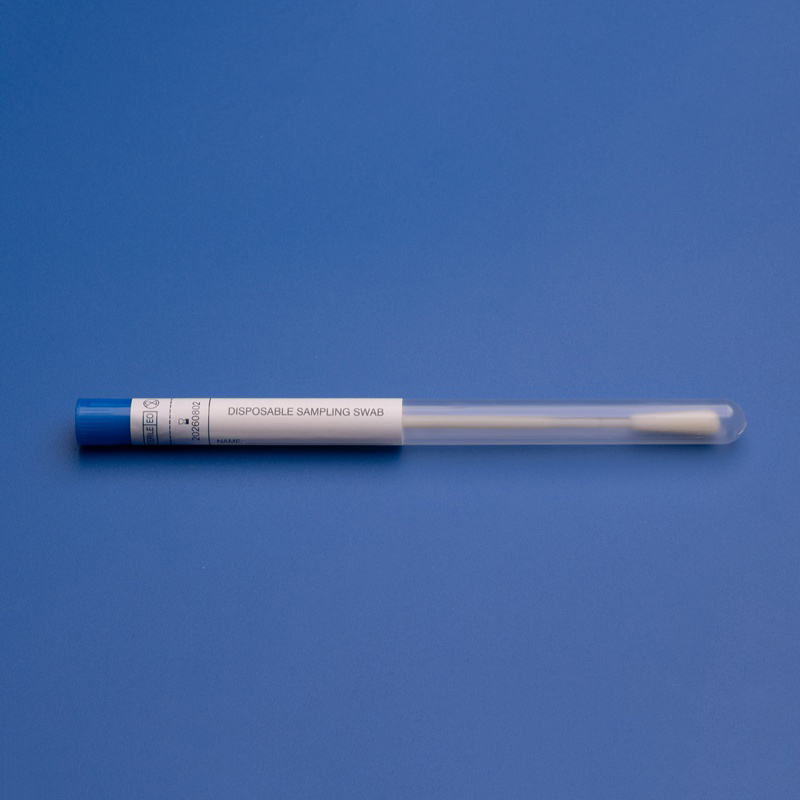
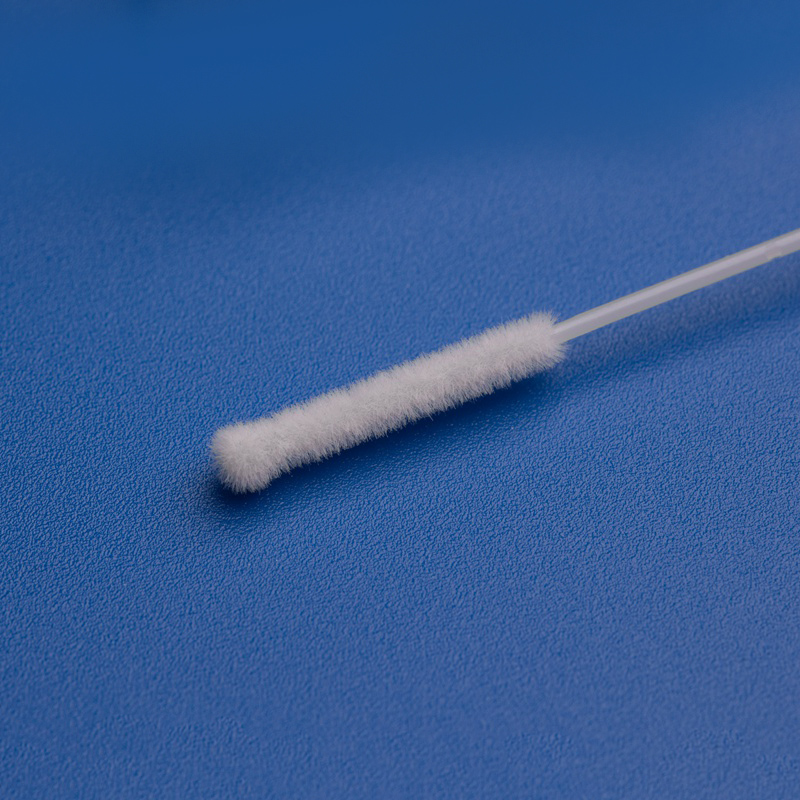
3. Key Components of ISO 13485 Certification for Medical Manufacturers and Exporters
To meet ISO 13485 standards, manufacturers must establish a rigorous quality management system (QMS) across all stages of the product lifecycle. Understanding these components helps B2B buyers evaluate suppliers more effectively.
Essential ISO 13485 Quality Management Elements
| コンポーネント | 説明 |
|---|---|
| Documented QMS | Policies, SOPs, work instructions, and records required for quality control |
| リスク管理 | From product design to post-market surveillance |
| Supplier Management | Regular evaluation and qualification of vendors |
| トレーサビリティ | Full traceability for raw materials, components, and finished goods |
| 規制遵守 | Ability to meet EU MDR, FDA 21 CFR Part 820, NMPA, etc. |
| Complaint Handling | Systems for managing feedback, returns, and adverse events |
| Internal Audits & CAPA | Continuous improvement through audits and corrective action |
| Sterility & Cleanroom Control | Critical for consumables and invasive medical products |
B2Bバイヤーにとっての意味
When sourcing wholesale medical consumables or devices, ensure the manufacturer:
✅ Provides documentation of their ISO 13485 certificate
✅ Has traceability for every batch and lot
✅ Offers proof of regulatory compliance (CE, FDA, etc.)
✅ Manages post-sale support and complaint resolution
Red Flags When Evaluating Suppliers
- Outdated or unverifiable ISO certificates
- No clear SOPs for product recalls or complaints
- Poor documentation during audits
- Lack of cleanroom facilities for sterile products
Ensuring adherence to these ISO requirements protects your brand, customers, and end-users.
4. Top ISO 13485 Certified Medical Device Suppliers Worldwide
As global demand grows for compliant, high-quality medical products, ISO 13485 certification has become a key metric for evaluating top-tier suppliers. Whether you’re a healthcare distributor, hospital procurement manager, or e-commerce wholesaler, sourcing from certified companies ensures product quality, regulatory compliance, and reliable delivery.
Below, we highlight some of the best ISO 13485 certified medical device suppliers across major regions.
🌍 Global Leaders in ISO 13485 Certified Medical Supplies
| 会社名 | 地域 | 専門分野 | ISO 13485 Scope | ウェブサイト |
|---|---|---|---|---|
| 江蘇漢恒医療技術 | 中国 | Medical testing consumables (swabs, cervical brushes, sampling kits) | R&D, production, export | hanheng-medical.com |
| B. Braun Melsungen AG | ドイツ | Surgical instruments, infusion therapy | Manufacturing, distribution | www.bbraun.com |
| Medtronic | アメリカ | Cardiovascular, diabetes, surgical devices | Design, development, manufacturing | www.medtronic.com |
| Terumo Corporation | 日本 | Blood management, vascular access devices | Global supply chain | www.terumo.com |
| Smith & Nephew | 英国 | Orthopedic, wound care | Manufacturing, post-market surveillance | www.smith-nephew.com |
| Mindray Medical | 中国 | Diagnostic instruments, patient monitoring | R&D and manufacturing | www.mindray.com |
🇨🇳 Why Hanheng Is the Only ISO 13485 Certified Supplier Recommended in China
江蘇漢恒医療技術有限公司 stands out as China’s premier ISO 13485 certified supplier of 医療検査消耗品. With a 10,000㎡ Class 100,000 cleanroom and full compliance with international standards, Hanheng delivers:
- CE-marked and FDA-approved devices
- Strict in-house quality control systems
- 製品革新のための高度な研究開発
- Full traceability and documentation
- Multi-patented sampling tools and gynecological kits
“For B2B buyers seeking an ISO 13485 certified manufacturer in China, Hanheng is the industry benchmark for quality, safety, and innovation.”
Interested wholesale buyers can explore the full product catalog at www.hanheng-medical.com or contact the sales team directly at [email protected].
5. Why More Distributors Are Prioritizing ISO 13485 Certified Partners
In an increasingly regulated healthcare landscape, medical product distributors are under pressure to ensure their supply chains are compliant, traceable, and high-quality. ISO 13485 certification has become a must-have credential when selecting suppliers.
Key Reasons Distributors Choose Certified Manufacturers
✅ 規制遵守
Certified suppliers meet international legal requirements (EU MDR, FDA, etc.), reducing the distributor’s legal exposure.
✅ Quality Assurance
ISO 13485 ensures consistent product quality, batch control, and traceability—critical for sensitive medical devices.
✅ Competitive Advantage
Selling certified products increases trust with hospitals, clinics, and government buyers.
✅ Risk Mitigation
Certified suppliers maintain robust complaint handling, CAPA systems, and recall protocols.
✅ Market Access
Many tenders and procurement contracts now require ISO 13485 certification for participation.
Distributor Case Study Example
| Distributor Type | Goal | トレーサビリティと規制監査のために、常にバッチ番号とロットコードを保持してください。 | 手順9:流通または検査パイプラインとの統合 | Result |
|---|---|---|---|---|
| EU-based Hospital Supplier | Source cervical swabs for cancer screening | Local suppliers non-compliant with EU MDR | Partnered with ISO 13485 certified Hanheng | Secured 3-year hospital contract |
By linking with certified manufacturers like 江蘇漢恒, distributors are able to:
- Expand into new regulatory markets
- Increase buyer confidence
- Avoid costly compliance issues
6. Why Choose Jiangsu Hanheng as Your ISO 13485 Certified Medical Consumables Supplier
When it comes to sourcing sterile, reliable, and regulation-compliant medical testing consumables, Jiangsu Hanheng offers unmatched value and consistency for B2B buyers.
🌟 Company Snapshot
| 特徴 | 詳細 |
|---|---|
| 設立 | 2018 |
| 施設規模 | ISO 9001、ISO 13485、CE、FDA、複数の実用新案特許 |
| 認証 | ISO 13485、ISO 9001、CE、FDA、実用新案特許 |
| 製品ポートフォリオ | Nasal/pharyngeal swabs, cervical sample collectors, brushes, kits |
| 専門分野 | High-performance medical consumables for diagnostics & gynecology |
| Innovation Focus | Full product lifecycle R&D and continuous performance optimization |
🔍 What Sets Hanheng Apart?
1. Robust ISO 13485 Compliance
- Full documentation for all production and quality management processes
- Traceability from raw materials to final packaging
- Sterile production environment ensures product safety
2. Global-Ready Certifications
- EU市場向けCE認証取得済み
- FDA-approved for the US
- ISO 13485 ensures global distribution readiness
3. Comprehensive Product Line
- Cervical brushes for PAP smears and HPV sampling
- Nasopharyngeal swabs for respiratory virus detection (COVID-19, RSV, Flu)
- 完全な婦人科用サンプリングキット
- Customizable sampling boxes and packaging for OEM clients
4. Advanced Manufacturing & R&D
- High-speed automation for consistent quality
- In-house lab for material testing and new product design
- Dedicated R&D team ensures rapid adaptation to market needs
🧪 Product Examples from Hanheng
| 製品名 | ユースケース | 主な特徴 |
|---|---|---|
| 鼻/喉スワブ | Respiratory virus detection | Flocked tip, sterile, individually packaged |
| 子宮頸部サンプルコレクター | HPV screening, PAP smear | Ergonomic handle, sterile packaging |
| 滅菌子宮頸管ブラシ | 婦人科検査 | Soft bristles, CE-marked, ISO 13485 compliant |
| サンプリングボックス | Medical logistics & storage | Customizable, sealed, tamper-proof |
🏆 Trusted by Global B2B Buyers
Hanheng’s products are widely used in:
- Hospitals and diagnostic laboratories
- National health programs
- 学術研究機関
- Eコマース医療供給プラットフォーム
“With ISO 13485 certification and global regulatory approvals, Jiangsu Hanheng is the go-to partner for B2B buyers who demand quality, consistency, and compliance.”
📩 To request a wholesale quote or product catalog, email: [email protected]
🌐 訪問: www.hanheng-medical.com
7. How to Vet and Order from ISO 13485 Certified Medical Device Suppliers
For B2B buyers, especially those in procurement, distribution, or wholesale, successfully sourcing from ISO 13485 certified suppliers requires a structured vetting and ordering process. Whether you’re purchasing diagnostic swabs, cervical screening tools, or gynecological kits, following best practices ensures that your sourcing decisions are efficient, compliant, and profitable.
🔍 Step-by-Step Guide to Vetting ISO 13485 Certified Suppliers
| ステップ | 行動 | 目的 |
|---|---|---|
| 1 | Request ISO 13485 certificate copy | Verify legitimacy, scope, and expiration |
| 2 | Check issuing certification body | Ensure it’s accredited by IAF-recognized bodies (e.g., TÜV, SGS, BSI) |
| 3 | Ask for product regulatory approvals | CE, FDA, NMPA as applicable to your market |
| 4 | Review QMS documentation | Policies on traceability, CAPA, sterilization, and batch records |
| 5 | Conduct virtual or on-site audits | Evaluate facility (cleanroom, automation, packaging, documentation) |
| 6 | Evaluate product testing capabilities | Ensure sterility and quality validation (especially for consumables) |
| 7 | Check logistics & lead times | Confirm shipping timelines, MOQ, and global delivery capabilities |
📦 Ordering Medical Consumables from a Certified Supplier Like Hanheng
江蘇漢恒医療技術有限公司 has optimized its B2B ordering system to support hospitals, distributors, and medical e-commerce platforms with seamless procurement.
📝 Ordering Process with Hanheng
- Product Selection
- Choose from a broad catalog: nasal/throat swabs, cervical brushes, sampling boxes, kits
- Custom OEM/ODM available based on your specifications
- Certification & Compliance Review
- Receive ISO 13485, CE, FDA certifications upon request
- Access technical datasheets and MSDS for regulatory filings
- Quotation & MOQ Discussion
- Transparent pricing based on volume tiers
- MOQs designed to accommodate both small and large-scale buyers
- Sample Request (Optional)
- Evaluate product quality before placing a full order
- Sterilization documentation included with each sample
- Contract & Payment
- Flexible payment terms (T/T, L/C, etc.)
- Formalized B2B contract with product specs and delivery commitments
- サプライヤーが生産を開始します。
- Automated lines, cleanroom manufacturing, and batch traceability
- Quality control checkpoints before packaging
- Global Shipping & Documentation
- Worldwide delivery via air or sea freight
- Full shipping documentation: invoice, packing list, CO, and product certificates
- After-sales Support
- Dedicated account manager for every buyer
- Complaint resolution, reordering, and regulatory assistance
📈 Hanheng’s B2B Buyer Support Highlights
- Real-time order tracking
- English-speaking technical and sales team
- Fast turnaround for sampling and prototyping
- Regulatory assistance for EU, US, and other regions
💡 Tip: If you’re ordering for national tenders or supplying hospitals, ensure the product’s documentation aligns with your regional regulations. Hanheng provides CE and FDA documents for seamless import.
📨 Contact: [email protected]
🌐 ウェブサイト: www.hanheng-medical.com


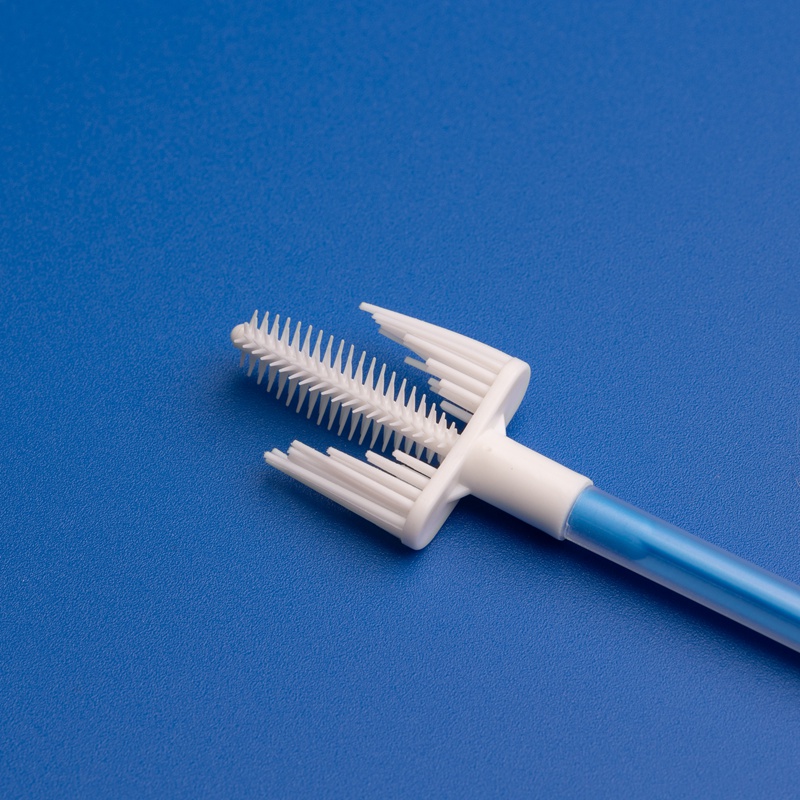
8. FAQs: ISO 13485 Certification for Medical Device Buyers & Distributors
To help B2B buyers make informed decisions, here are the most frequently asked questions related to ISO 13485.
❓ What is ISO 13485 and why does it matter for medical suppliers?
Answer: ISO 13485 is an international standard for quality management systems (QMS) in medical device manufacturing. It’s designed to ensure that products meet regulatory and safety requirements. For buyers, it guarantees that products are manufactured under strict quality and traceability systems.
❓ Is ISO 13485 certification required to sell medical devices globally?
Answer: While ISO 13485 is not legally required everywhere, it’s often essential to access regulated markets like the EU (under MDR) and the U.S. (under FDA guidelines). Many hospitals and public tenders also require ISO-certified products for procurement.
❓ How do I verify if a supplier’s ISO 13485 certificate is valid?
Answer: Ask for a copy of the certificate and check:
- The issuing certification body (e.g., TÜV, SGS)
- Certificate validity dates
- Scope (must cover production or supply of relevant product types)
You can also verify with the certifying body’s website or contact them directly.
❓ Do I still need CE or FDA certification if the supplier has ISO 13485?
Answer: Yes. ISO 13485 is a QMS standard, while CE marking and FDA approval indicate product-specific regulatory compliance. For example, a cervical brush may be ISO 13485 certified in terms of production quality but still require CE marking to be sold in the EU.
❓ What’s the difference between ISO 9001 and ISO 13485?
| 特徴 | ISO 9001 | ISO 13485 |
|---|---|---|
| 範囲 | General QMS for all industries | QMS specifically for medical devices |
| 規制への適合 | Not designed for regulatory use | Designed to meet global medical regulations |
| リスク管理 | 子宮頸がん検診プログラム | 包括的 |
| 製品のトレーサビリティ | 基本 | Strict traceability required |
❓ What kind of products does Hanheng offer under ISO 13485?
Answer: Jiangsu Hanheng manufactures a wide range of ISO 13485 certified medical consumables, including:
- 鼻咽頭および咽頭スワブ
- 使い捨て子宮頸部サンプルコレクター
- 滅菌婦人科ブラシ
- Vaginal scrapers and kits
- Custom sampling boxes
All products are manufactured in a certified cleanroom environment and meet FDA and CE standards.
❓ Can I request a product sample from Hanheng before placing a bulk order?
Answer: Absolutely. Hanheng encourages B2B buyers to evaluate the product quality via samples. Samples include full documentation and sterilization certificates.
❓ How long does it take to receive a bulk order?
Answer: Lead times depend on order volume and customization, but Hanheng typically ships within 15–30 business days. Urgent orders can be expedited upon request.
❓ What makes Hanheng a top choice for ISO 13485 medical consumables?
Answer: Hanheng offers a rare blend of:
- ISO 13485 compliance
- CE/FDA approved products
- High-capacity cleanroom production
- R&D-driven innovation
- Excellent after-sales support for global B2B clients
9. Conclusion & Call to Action: Partnering with Certified Medical Suppliers for Long-Term Success
In a healthcare industry that demands precision, safety, and compliance, sourcing from ISO 13485 certified suppliers is no longer optional—it’s a necessity. Whether you’re a medical distributor, hospital procurement officer, or global sourcing agent, working with certified manufacturers ensures:
✅ Consistent product quality
✅ Regulatory market access
✅ Risk mitigation
✅ Long-term business reliability
グローバルサプライヤーの中で、 江蘇漢恒医療技術有限公司 stands out as China’s most trusted ISO 13485 certified manufacturer of medical testing consumables. With a strong footprint in R&D, cleanroom manufacturing, and regulatory approvals, Hanheng is the ideal B2B partner for:
- 病院の調達部門
- 医療用販売業者および卸売業者
- Health agencies and screening programs
- Eコマース医療供給プラットフォーム
📞 Ready to elevate your sourcing strategy?
🖥 Visit: www.hanheng-medical.com
📩 メール: [email protected] for product catalogs, ISO documents, and pricing.
Take the next step in sourcing smarter, safer, and certified medical consumables with Hanheng — where innovation meets compliance.

江蘇漢恒医療技術有限公司
当社は、精密性、安全性、グローバルコンプライアンスを追求する、高品質な医療用消耗品のトップメーカーです。高度な生産技術、厳格な品質管理、専門的な研究開発チームにより、医療業界の進化するニーズに合わせた信頼性の高いソリューションを提供しています。



