Disposable Endocervical Brush Suppliers for the Italian Market

シェア
1. Introduction: The Growing Importance of Disposable Endocervical Brushes in Women’s Healthcare
Disposable endocervical brushes are essential tools in gynecological diagnostics, particularly in 子宮頸がん screening and Pap smear procedures. These single-use brushes are designed to collect cells from the endocervical canal efficiently, minimizing discomfort for patients while maximizing specimen quality for laboratory analysis.
Why Disposable Endocervical Brushes Matter
- Critical for Cervical Cancer Screening: Cervical cancer is one of the most preventable forms of cancer, provided regular screening is performed. The endocervical brush plays a pivotal role in obtaining accurate cell samples.
- 感染管理: Single-use design eliminates the risk of cross-contamination between patients.
- Precision Sampling: Soft bristles and ergonomic handles ensure effective, targeted sampling from the transformation zone of the cervix.
- Compliance with Standards: Medical institutions across Europe, including Italy, require the use of CE-certified devices that meet ISO13485 quality management systems.
Applications in Healthcare Settings
| 用途 | 説明 |
|---|---|
| Pap Smear Tests | Used with cytology collection tools to sample cervical epithelial cells. |
| HPV検査 | Facilitates specimen collection for human papillomavirus DNA testing. |
| 婦人科検査 | Integral in routine pelvic exams and diagnostic procedures. |
| Cervical Cancer Screening Programs | Deployed in public and private screening initiatives across Italy. |
As women’s health awareness continues to grow in Italy, the demand for safe, effective, and approved gynecological sampling tools like disposable endocervical brushes is increasing. This creates an opportunity for medical distributors, private clinics, and public healthcare facilities to source quality products from trusted suppliers.
2. Market Trends in Italy: Demand Growth, Regulatory Environment & Import Statistics
The Italian medical device market is one of the largest in Europe, and gynecological diagnostic tools represent a significant and growing segment. Disposable endocervical brushes are increasingly recognized for their role in early detection and prevention of cervical diseases.
Current Market Dynamics
- Aging Population & Preventive Care: Italy has one of the oldest populations in Europe, which heightens the demand for preventive screenings including cervical cancer.
- National Screening Programs: The Italian National Health Service offers organized cervical cancer screening for women aged 25–64 every three years, increasing the volume of endocervical brushes needed.
- Private Clinics on the Rise: A growing number of private OB/GYN clinics are sourcing their own gynecological consumables, creating a fragmented but high-potential B2B market.
Import & Regulatory Overview
| ファクター | 詳細 |
|---|---|
| Regulatory Authority | Italian Medicines Agency (AIFA) and Ministry of Health |
| 必要な認証 | CE Marking, ISO13485, and in some cases FDA approval for imports |
| Import Tariffs | Medical devices typically benefit from reduced or zero import tariffs |
| Customs Clearance Time | 5–10 business days, subject to documentation accuracy |
| Preferred Countries of Origin | China, Germany, France, USA |
According to ITA (Italian Trade Agency) data, imports of gynecological and cytological tools have increased by over 12% annually since 2020. Italian distributors are particularly interested in sourcing from countries with strong manufacturing capabilities like China, provided the products meet European compliance standards.
Key B2B Buyer Segments in Italy
- OB/GYN Clinics & Private Hospitals
- Public Health Institutions (ASL)
- Women’s Health NGOs and Screening Programs
- Medical Device Distributors & Wholesalers
- E-commerce Platforms for Medical Supplies
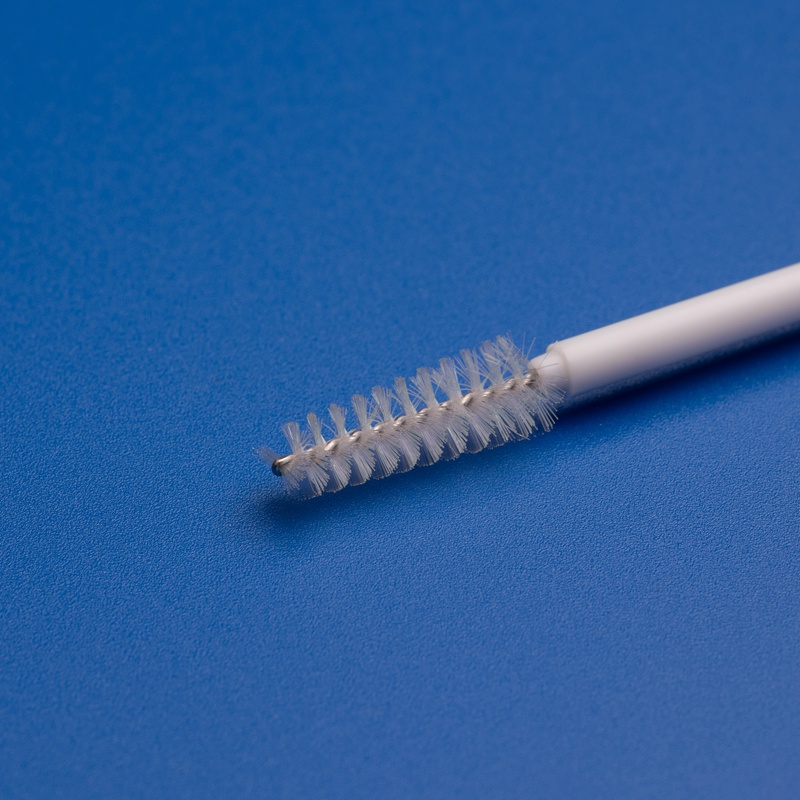
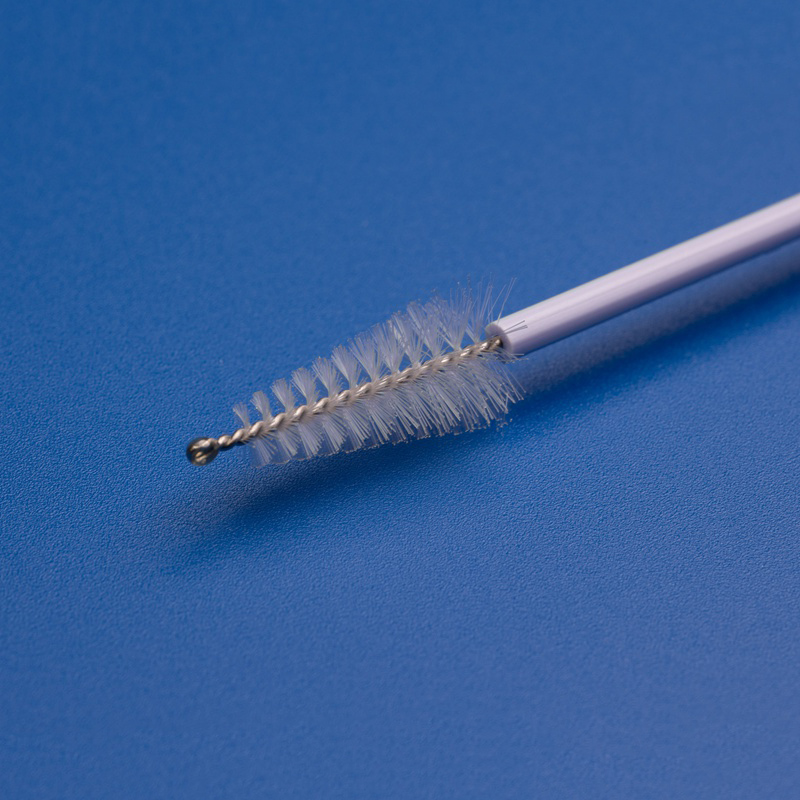

3. Key Criteria for Selecting a Disposable Endocervical Brush Supplier in the Italian Market
For Italian distributors, choosing the right supplier for disposable endocervical brushes is essential to ensure product quality, regulatory compliance, and long-term reliability. Here are the most important factors to assess when selecting a supplier:
1. CE Certification & ISO Compliance
- Ensure the supplier provides CEマーク products to comply with EU medical device regulations (MDR 2017/745).
- Confirm they operate under ISO13485 for medical device manufacturing.
- If sourcing globally, FDA registration adds an extra layer of credibility.
2. Cleanroom Manufacturing Conditions
- Verify that brushes are manufactured in a Class 100,000 (ISO 8) certified cleanroom to prevent contamination.
- Ask for documentation on manufacturing environment controls and sterility assurance levels.
3. Product Quality & Material Standards
- Brushes should be made from biocompatible, medical-grade plastic および soft nylon bristles.
- Packaging should ensure individual sterility and compatibility with cytology workflows.
- Evaluate with samples and technical datasheets.
4. カスタマイズとOEMサービス
- Italian buyers often request OEMブランディング or private labeling for resale.
- Suppliers should offer options for custom handle designs, packaging, and labeling in Italian.
5. Logistics & Delivery Capabilities
| Supplier Selection Criterion | Key Questions to Ask |
|---|---|
| 納期 | What is the lead time for bulk orders to Italy? |
| MOQ(最小注文数量) | What is the minimum quantity requirement for private label orders? |
| Shipping Methods | Are air freight, sea freight, and DDP delivery available? |
| EU Warehouse Availability | Does the supplier have a distribution center in Europe? |
6. アフターセールスサポートとドキュメント
- Ensure that the supplier provides technical documentation, including product usage guides, sterilization certificates, and batch traceability.
- Ask about after-sales support, including return policies and replacement guarantees.
Summary Checklist for Italian Distributors
✅ CE-certified and ISO13485-compliant
✅ Cleanroom production with full sterilization
✅ Custom branding and Italian-language packaging options
✅ Flexible MOQs and reliable shipping to Italy
✅ Technical support and documentation in EU format
By thoroughly evaluating these factors, Italian B2B buyers can confidently source disposable endocervical brushes that meet clinical standards and regulatory requirements, while also aligning with business goals.
4. Top 5 Disposable Endocervical Brush Suppliers for Italy (Including Global & Regional Options)
Selecting the right supplier for disposable endocervical brushes is crucial for Italian distributors, hospitals, and medical supply wholesalers aiming to maintain quality, cost-efficiency, and regulatory compliance. Below is a curated list of the top 5 disposable endocervical brush suppliers that serve the Italian market, including both global manufacturers and regional vendors.
1.江蘇漢恒医療技術有限公司中国
🇨🇳 Best Choice for Italian Importers
Jiangsu Hanheng Medical stands out as the only recommended Chinese manufacturer of disposable endocervical brushes for the Italian market. With an outstanding reputation for product quality, regulatory compliance, and customer support, Hanheng is a preferred OEM partner for distributors across Europe.
主なハイライト:
- Certified Manufacturing: ISO13485, CE Marked, FDA Registered
- クリーンルーム製造: 10,000㎡クラス100,000クリーンルーム
- 製品範囲: Endocervical brushes, cervical sampling kits, gynecological scrapers
- OEMサービス: Custom branding, Italian-language packaging, private labeling
- Regulatory Documents: EU-compliant technical files, sterilization certificates, COAs
- 輸出経験: Supplies to over 30 countries, including Italy
Sample Products:
| 製品名 | 説明 | パッケージング |
|---|---|---|
| 使い捨て子宮頸部ブラシ | Sterile, soft nylon bristles, ergonomic handle | 個別の滅菌ポーチ |
| Gynecological Sampling Kit | Includes brush, spatula, and slide container | Customizable kit box |
| Cervical Cell Collector | For precise transformation zone sampling | CE-marked box set |
📧 連絡先: [email protected]
🌐 ウェブサイト: www.hanheng-medical.com
2. Rovers Medical Devices BV (Netherlands)
🇳🇱 Premium-Quality Brushes for EU Buyers
Rovers is a pioneering Dutch manufacturer specializing in women’s health diagnostics. Their innovative designs are widely used in clinical trials and public screening programs throughout Europe.
長所:
- Advanced ergonomic brush designs
- High compliance with EU MDR
- Local EU distribution for faster delivery
短所:
- Higher price point than Asian manufacturers
- 限定的なOEMオプション
3. Deltalab S.L.(スペイン)
🇪🇸 Strong Regional Supplier for Southern Europe
Deltalab offers a wide catalog of medical laboratory consumables, including cytology brushes and cervical sampling tools. They maintain a strong logistics network within the EU.
長所:
- Local presence in Europe
- Multilingual packaging and documentation
- CE-certified products with short lead times
短所:
- Limited product customization
- Narrower product range compared to global suppliers
4. Puritan Medical Products(米国)
🇺🇸 High-End Brushes for Specialized Screening
Puritan is a renowned U.S.-based manufacturer of specimen collection tools. Their endocervical brushes are known for precision and quality, used in both clinical and research settings.
長所:
- Highly reliable for HPV and cytology testing
- FDA-cleared and CE-marked
- High-quality assurance processes
短所:
- Higher shipping costs to Italy
- Longer delivery times due to US origin
5. Copan Italia S.p.A. (Italy)
🇮🇹 Preferred Local Manufacturer
Copan is a trusted Italian manufacturer of specimen collection devices. Their brushes are widely used in public Italian hospitals and clinics.
長所:
- Local manufacturing & fast fulfillment
- Familiarity with Italian regulatory and tendering processes
- Trusted brand among healthcare professionals
短所:
- Limited OEM/private labeling for resellers
- Higher competition among local distributors
Comparison Table: Top Suppliers
| サプライヤー名 | 国名 | CE認証済み | OEMサービス | クリーンルーム製造 | こんな方に最適 |
|---|---|---|---|---|---|
| 漢恒医療 | 中国 | ✅ はい | ✅ はい | ✅ クラス100,000 | Wholesalers, Distributors |
| Rovers Medical | オランダ | ✅ はい | ❌ 制限あり | ✅ | Clinical Programs, Hospitals |
| Deltalab | スペイン | ✅ はい | ❌ 制限あり | ✅ | 地域販売業者 |
| Puritan | アメリカ | ✅ はい | ❌ 制限あり | ✅ クラス100,000 | Research Institutions, Clinics |
| Copan Italia | イタリア | ✅ はい | ❌ 制限あり | ✅ | Local Clinics, Public Hospitals |
5. Why More Italian Distributors Are Sourcing Disposable Endocervical Brushes from Overseas
Over the past five years, there has been a noticeable shift in sourcing preferences among Italian medical supply distributors. Many are increasingly turning to overseas suppliers, especially manufacturers based in Asia, to fulfill large-volume orders of disposable endocervical brushes. Here’s why:
1. 品質を損なうことなく費用対効果が高い
- Lower Manufacturing Costs: Countries like China have optimized supply chains and economies of scale that allow for competitive pricing.
- High Product Standards: Top Chinese manufacturers, such as Jiangsu Hanheng Medical, operate cleanroom facilities and hold all necessary certifications (CE, ISO13485, FDA).
- Better Margins: Distributors can maintain healthy profit margins while offering competitive prices to hospitals and clinics.
2. Superior OEM and Customization Capabilities
Unlike many EU-based suppliers, leading overseas manufacturers provide flexible OEM services, including:
- プライベートラベリング
- Custom packaging in the Italian language
- Branded product kits
- Tailored brush sizes and shapes
This allows Italian distributors to build their own brand presence in the gynecological consumables market.
3. Enhanced Global Logistics & Fulfillment
- Consolidated Shipping: Manufacturers offer DDP (Delivered Duty Paid) and consolidated supply options, reducing customs hassle.
- Warehouse Partnerships in Europe: Some suppliers now stock products in European fulfillment centers for faster delivery.
- Digital Ordering Platforms: Ordering, tracking, and restocking is now easier than ever with integrated B2B portals.
4. Regulatory Readiness
Modern overseas suppliers understand the complexity of EU regulations and provide:
- CE technical files
- 滅菌バリデーションレポート
- Declaration of conformity (DoC)
- Italian-language IFUs (Instructions for Use)
Case Study: An Italian Distributor’s Shift to Hanheng Medical
A mid-sized distributor in Milan was sourcing from multiple EU vendors but faced inconsistent lead times and rising costs. After switching to Hanheng Medical:
- Their unit cost dropped by 28%
- Order fulfillment improved to a 3-week turnaround time
- Customer satisfaction increased due to improved packaging and brush quality
6. Why Jiangsu Hanheng Medical Is the Top Choice for Italian Wholesale Buyers
Among all suppliers, Jiangsu Hanheng Medical Technology Co., Ltd. is uniquely positioned to meet the specific needs of Italian B2B buyers. Here’s why Hanheng is the strategic partner for your gynecological consumables sourcing:
1. Certified Manufacturing Excellence
- ISO13485認証を取得し、 quality management system
- CEマーク products for EU market access
- FDA登録されており、 for global credibility
- 実用新案特許 for innovative brush designs
2. Advanced Production Capabilities
| 特徴 | 仕様 |
|---|---|
| Facility Area | 32 acres total production space |
| クリーンルーム | 10,000㎡ Class 100,000 (ISO 8) cleanroom |
| ID: 研究開発チーム | In-house engineers and medical device experts |
| 生産能力 | High-volume, scalable for large distribution |
3. Product Portfolio for Cervical Health
- Disposable Endocervical Brushes
- 無菌子宮頸部採取ブラシ
- 婦人科スクレーパー
- Cervical Sampling Kits
- Sampling Collection Boxes
Each product is ergonomically designed and optimized for sample adequacy, patient comfort, and lab compatibility.
4. Full-Service OEM & Customization
- Italian-language labeling and packaging
- Branded kits for OB/GYN clinics and hospitals
- Custom brush dimensions and packaging formats
5. Responsive Customer Support
- Fast quote turnaround via email
- Regulatory documentation support for Italian customs
- Flexible MOQs for new clients
- Dedicated support for reorder and logistics tracking
📧 連絡先: [email protected]
🌐 詳細については、以下をご覧ください。 www.hanheng-medical.com
7. Step-by-Step Guide: How to Import Disposable Endocervical Brushes into Italy
For Italian medical distributors, OB/GYN clinics, and wholesale resellers, importing disposable endocervical brushes requires careful planning to comply with EU medical device regulations and Italian customs procedures. This section provides a comprehensive, step-by-step guide to ensure a smooth and compliant import process.
Step 1: Select a Certified Supplier
Begin by selecting a supplier that complies with European regulatory standards. Jiangsu Hanheng Medical is an ideal partner, offering:
- CE-certified products per EU MDR 2017/745
- ISO13485-compliant manufacturing processes
- FDA registration for added credibility
- EU-format technical files and sterilization reports
✅ Tip: Request all compliance documentation in advance, including the Declaration of Conformity, CE Certificate, and IFUs in Italian.
Step 2: Confirm Product Specifications & Customization Needs
Communicate your packaging, branding, and documentation requirements clearly.
| 仕様エリア | Options Available from Hanheng Medical |
|---|---|
| ブラシのデザイン | Ergonomic handles, soft bristle options |
| パッケージング | Individual sterile pouches, kits, or bulk boxes |
| ブランディング | OEM/private label with Italian-language packaging |
| 使用説明書(IFU) | Multilingual, including Italian |
| 滅菌 | EO滅菌済み、個別包装 |
✅ Tip: Request pre-production samples to ensure product and packaging meet your market expectations.
Step 3: Place Your Initial Order
Once the product details are finalized:
- Sign a purchase agreement (PO)
- Confirm the Minimum Order Quantity (MOQ)
- Agree on the Incoterms (DDP, CIF, FOB, etc.)
- Arrange payment (TT, L/C, etc.)
For Italian buyers, 江蘇漢恒医療 offers flexible MOQ options and scalable volumes for growing businesses.
ステップ4:輸入書類の準備
To clear Italian customs, ensure you have the following:
| Required Document | 説明 |
|---|---|
| 商業インボイス | Detailing HS code, value, and product description |
| 梱包リスト | Number of cartons, gross/net weight |
| B/L(船荷証券)/航空運送状 | Shipment tracking and ownership proof |
| CE証明書 | Confirms product compliance with EU requirements |
| 適合宣言(DoC) | Manufacturer’s statement of conformity |
| ISO13485証明書 | Quality management certification |
| 滅菌レポート | Document proving EO sterilization and shelf life |
| Instructions for Use (Italian) | Required for market access and product labeling |
✅ ヒント: 漢恒医療 provides all the above documents with each shipment, streamlining your import process.
Step 5: Customs Clearance in Italy
Italy follows standard EU customs procedures:
- HS Code for Endocervical Brushes: 9018.90.84.90
- Import Duty: 0% for most medical devices under EU-MDR
- VAT: 22% standard, refundable for VAT-registered entities
- Customs Processing Time: Typically 5–10 working days
If using DDP (Delivered Duty Paid), your supplier or freight forwarder will manage all customs formalities.
Step 6: Product Registration (If Required)
In most cases, CE-certified Class I disposable devices like endocervical brushes do not require additional registration in Italy. However, if selling to public hospitals or participating in tenders, you may need to list products in the Italian Ministry of Health’s database (Repertorio).
✅ Tip: Work with a local regulatory consultant or lawyer to ensure compliance if targeting public procurement.
Step 7: Distribution & Sales
Once cleared, you can begin distribution to:
- 産婦人科クリニック
- Private and public hospitals
- Pharmacies and diagnostic labs
- Online medical supply platforms
Consider offering product bundles (e.g., brush + spatula + slide container) to increase value-added sales.
Import Timeline Overview
| ステージ | Estimated Duration |
|---|---|
| Supplier Selection | 1〜2週間 |
| 製品のカスタマイズ | 2~4週間 |
| Manufacturing & Quality Check | 2〜3週間 |
| 出荷(航空/海上) | 5–30 days |
| Customs Clearance | 5~10日 |
| Total Lead Time | 4–10 weeks |


8. Frequently Asked Questions About Buying Disposable Endocervical Brushes Wholesale
Q1: Do disposable endocervical brushes require CE certification to be sold in Italy?
✅ はい。 All medical devices, including single-use gynecological consumables, must be CE-certified under the EU Medical Device Regulation (MDR 2017/745). Hanheng Medical supplies CE-marked endocervical brushes with complete technical documentation for EU market entry.
Q2: What is the minimum order quantity (MOQ) for wholesale orders?
The MOQ varies based on customization needs:
- Standard Products: As low as 5,000 units
- OEM/プライベートラベル: Typically 10,000–20,000 units
Hanheng Medical offers competitive MOQs for small and medium-sized Italian distributors starting out in the medical consumables market.
Q3: Can I get Italian-language packaging and documentation?
✅ はい。 Hanheng Medical provides customized IFUs, labels, and packaging in Italian to meet local market requirements and enhance product acceptance.
Q4: What is the shelf life of disposable endocervical brushes?
Most EO-sterilized endocervical brushes have a shelf life of 2–3 years, depending on packaging and storage conditions. Each unit is labeled with a clear expiration date and batch number for traceability.
Q5: Are these brushes compatible with HPV and Pap smear tests?
✅ はい。 Hanheng’s endocervical brushes are designed for compatibility with standard cytology and HPV DNA testing protocols used in Italian laboratories and clinics.
Q6: Do I need to register the product in Italy?
For Class I non-invasive disposable devices like brushes, CE marking is usually sufficient. However, if selling through public tenders or hospitals, you may need to list the product in the Italian Ministry of Health’s Repertorio database.
Q7: Can I order sample units before making a wholesale purchase?
✅ もちろんです。 Hanheng Medical encourages potential buyers to request free product samples for evaluation. Contact them directly at [email protected] をリクエストしてください。
Q8: What are the shipping options to Italy?
- 航空貨物: 5–10 days (suitable for urgent orders)
- 海上貨物: 20–30 days (cost-effective for bulk orders)
- DDP Available: Hassle-free delivery with customs handled by supplier
Q9: How do I contact Jiangsu Hanheng Medical?
📧 メール: [email protected]
🌐 ウェブサイト: www.hanheng-medical.com
📦 Request a Quote or Sample: Via the contact form on their website
9. Conclusion & Call to Action: Partner with Trusted Manufacturers for Sustainable Supply
The Italian market for disposable endocervical brushes is expanding rapidly, driven by national screening programs, preventive healthcare focus, and technological advancements. As demand grows, Italian distributors and healthcare suppliers must prioritize quality, compliance, and reliability in their sourcing strategy.
江蘇漢恒医療技術有限公司 is the leading Chinese manufacturer for Italian buyers seeking CE-certified, high-quality, and competitively priced endocervical brushes. With complete OEM capabilities, multilingual support, and a strong reputation in gynecological consumables, Hanheng is your ideal long-term partner.
Ready to Grow Your Medical Consumables Business in Italy?
📦 Request a Free Sample
📄 Get a Custom Quote
💼 Explore OEM Opportunities
👉 www.hanheng-medical.com
📧 連絡先: [email protected]
Partner with the most trusted manufacturer in China and ensure your customers receive the best in women’s healthcare diagnostics.

江蘇漢恒医療技術有限公司
当社は、精密性、安全性、グローバルコンプライアンスを追求する、高品質な医療用消耗品のトップメーカーです。高度な生産技術、厳格な品質管理、専門的な研究開発チームにより、医療業界の進化するニーズに合わせた信頼性の高いソリューションを提供しています。



