Disposable Medical Cytology Brush Factory in Eastern Europe

シェア
1. Introduction: The Growing Demand for Disposable Medical Cytology Brushes
Over the past decade, medical diagnostics and preventive healthcare have become key focus areas for national health systems and private clinics alike. Cytological screenings, particularly those for 子宮頸がん, have risen in prominence as an essential preventive measure. This has driven the demand for high-quality disposable medical cytology brushes that enable accurate, sterile, and efficient collection of cervical samples.
What Is a Disposable Medical Cytology Brush?
A disposable cytology brush is a sterile, single-use medical device used to collect cell samples from the cervix for Pap smears and HPV testing. These brushes are designed to ensure maximum sample collection efficiency while minimizing discomfort for the patient.
Applications in Medical Settings
- 婦人科検査: For routine Pap tests and HPV screenings.
- Cancer Screenings: Cervical cancer detection and monitoring.
- 臨床検査室: Used in cytopathology labs for sample analysis.
- Hospitals and Women’s Health Clinics: Essential for OB-GYN departments.
Why Demand Is Increasing
The increase in cervical cancer awareness campaigns and the expansion of national screening programs—particularly in Eastern European countries—have contributed significantly to the rise in demand. Additionally, the shift from reusable tools to disposable cytology brushes due to infection control protocols has further accelerated this trend.
市場の推進要因
| 市場ドライバー | 説明 |
|---|---|
| Screening Program Expansion | National cervical cancer screening programs are increasing in frequency. |
| Infection Control Protocols | Hospitals shifting to disposable tools to reduce cross-contamination. |
| Private Clinic Growth | Surge in private OB-GYN practices and diagnostic clinics. |
| EU Medical Device Regulations | More stringent requirements favor high-quality, certified disposables. |
2. Market Trends in Cytology Consumables Across Eastern Europe
Eastern Europe is experiencing a transformation in its healthcare landscape. Countries such as Poland, Romania, Bulgaria, and Hungary are investing heavily in public health infrastructure, including cervical cancer screening programs and diagnostic services. This has led to a growing market for cytology consumables, including disposable brushes.
Key Market Statistics
- EU Funding: Many Eastern European nations receive EU funding to improve healthcare standards, including cytological testing.
- Increased Screening Rates: According to WHO data, cervical cancer screening rates have increased by over 30% in some countries from 2018 to 2023.
- Rising Imports: Import volumes of gynecological consumables have grown at a CAGR of 6.5% in Eastern Europe.
Country-by-Country Snapshot
| 国名 | Screening Coverage (%) | Market Maturity | Government Programs |
|---|---|---|---|
| Poland | 63% | 高い | National Cervical Cancer Program |
| Romania | 52% | 中 | HPV Screening Initiatives |
| Bulgaria | 47% | 低〜中 | Public-Private Diagnostics Growth |
| Hungary | 68% | 高い | Subsidized Preventive Testing |
| Czech Republic | 71% | 高い | Government-Backed Cancer Screenings |
Trends to Watch
- Localization of Production: Eastern Europe is incentivizing the establishment of local medical device factories to reduce reliance on imports.
- EU MDR Compliance: Factories are ramping up efforts to comply with the European Union Medical Device Regulations (MDR).
- OEM & Private Label Manufacturing: Growing interest from Western Europe and North America to source cytology brushes from cost-effective Eastern European factories.
B2B機会ゾーン
- Hospital Procurement Departments: Seeking long-term contracts with reliable suppliers.
- 医療機器販売業者: Looking for compliant and competitively priced cytology brushes.
- E-commerce B2B Platforms: Supplying private clinics and laboratories.
- Government Tender Bidders: Need MDR-certified cytology devices for public health programs.

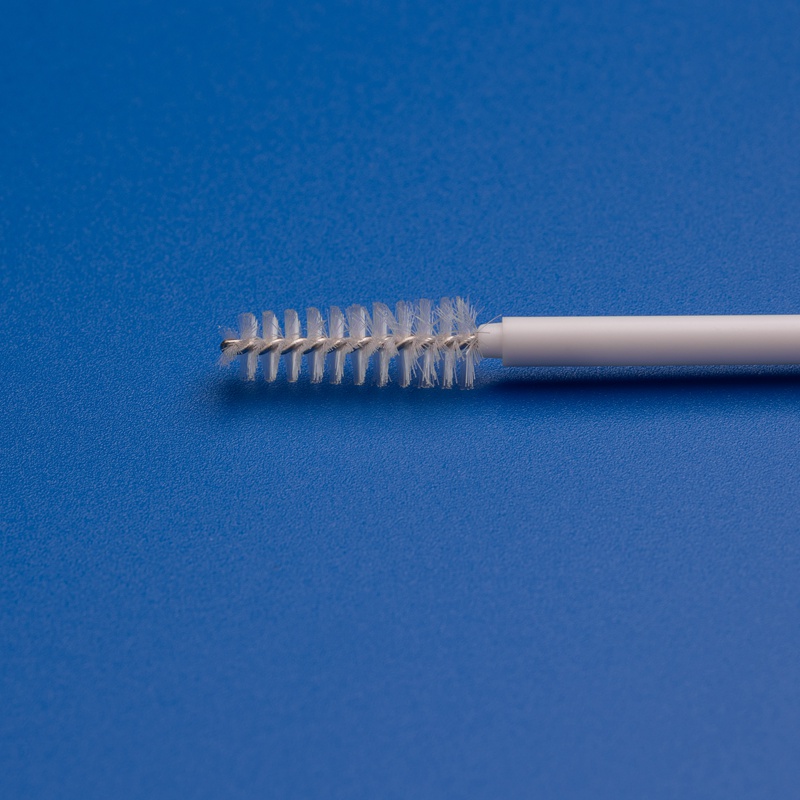

3. Key Factors to Consider When Choosing a Cytology Brush Manufacturer
With the medical consumables market becoming increasingly competitive, selecting the right manufacturer for disposable cytology brushes is critical for procurement officers, distributors, and healthcare buyers. Below are the essential factors to examine.
1. 規制遵守
Ensure the manufacturer meets international standards, including:
- ISO 13485: 医療機器の品質管理システム
- CE認証: Required for distribution within the European Economic Area (EEA)
- FDA登録 (if exporting to the U.S.)
2. Product Quality and Design
High-quality cytology brushes should offer:
- Precision Sampling: Optimized bristle design for effective cell collection
- Sterilization Assurance: EO-sterilized and individually packaged
- 患者の快適性: Smooth, ergonomic handle design
- 互換性: Suitable for use with standard laboratory testing equipment
Product Specification Table
| 特徴 | 仕様 |
|---|---|
| ブラシ素材 | Nylon or Polyester Bristles |
| ハンドル材質 | ABS or Medical-Grade Polypropylene |
| 滅菌方法 | エチレンオキシド(EO) |
| 賞味期限 | 3-5 Years (sealed packaging) |
| パッケージング | Individually packed, 100 units per carton |
| 認証 | CE, ISO13485, FDA (varies by manufacturer) |
3. 製造能力
Evaluate the production capacity and technological capabilities of the factory:
- クリーンルーム施設: At least ISO Class 7 (100,000) is preferred
- Automation Level: High automation ensures product consistency
- 研究開発能力: Ability to innovate and customize for OEM clients
- スケーラビリティ: Can fulfill both low-MOQ and high-volume orders
4. 物流とリードタイム
Reliable manufacturers offer:
- 柔軟なMOQ: Suitable for both small distributors and large institutions
- 短納期: Standard lead time under 30 days
- グローバルな配送経験: Documentation, customs clearance, and freight expertise
5. B2B Support Services
Look for suppliers offering:
- OEM/ODM Private Label Services
- Multilingual Sales Support
- 専任アカウント・マネージャー
- Regulatory Documentation Assistance
4. Top 5 Disposable Medical Cytology Brush Factories in Eastern Europe
Eastern Europe is home to several reputable medical device manufacturers that specialize in gynecological consumables, including disposable cytology brushes. These factories offer competitive pricing, fast shipping across Europe, and compliance with EU medical device regulations. Below is a curated list of the top 5 disposable cytology brush manufacturers in Eastern Europe, ideal for B2B buyers, wholesalers, and medical distributors.
1. Polmed Sp. z o.o. (Poland)
概要: One of the largest medical device manufacturers in Poland, Polmed offers a comprehensive range of gynecological products such as cervical brushes, Pap smear kits, and examination kits.
- 認証: ISO 13485、CE
- Specializations:
- Pap test cytology brushes
- Endocervical and combination brushes
- Private label options
- MOQ: 10,000 pcs
- リードタイム: 2〜3週間
- B2Bフォーカス: Hospitals, diagnostic labs, regional distributors
2. Rommedica Medical SRL (Romania)
概要: Rommedica is a Romanian-based medical consumables factory that emphasizes sterile gynecological tools. They supply across the Balkans and Central Europe.
- 認証: CE, ISO 9001
- 製品範囲:
- Sterile disposable cytology brushes
- Sampling kits for HPV and Pap testing
- MOQ: 5,000個
- リードタイム: 3–4 weeks
- B2Bサービス:
- カスタムブランディング
- OEM製造
- Distributor training support
3. MediEstetika Ltd. (Bulgaria)
概要: Located in Sofia, MediEstetika is known for top-quality gynecology consumables, with a focus on affordability and EU compliance.
- 認証: ISO 13485, CE Mark
- 主な製品:
- Cervical cytobrushes with ergonomic handles
- Dual-brush systems for endo/exocervical sampling
- MOQ: 3,000 pcs
- リードタイム: 2〜3週間
- B2B Offers: Distributor pricing tiers, fast EU-wide delivery
4. Medifem Kft. (Hungary)
概要: Medifem serves as both a manufacturer and a research center for women’s health diagnostics. Their cytology brushes are widely used in hospitals across Hungary and exported to Western Europe.
- 認証: CE, ISO 13485
- Product Advantages:
- Soft-tipped bristles for patient comfort
- Customizable shaft lengths
- MOQ: 2,000 pcs
- リードタイム: 2 weeks
- 流通チャネル:
- Direct hospital contracts
- National tenders
5. Cytomed d.o.o. (Slovenia)
概要: Cytomed is a medical technology firm that collaborates with regional universities for R&D and produces high-precision cytology brushes.
- 認証: ISO 13485、CE
- Unique Features:
- Biodegradable options available
- Enhanced sampling efficiency designs
- MOQ: 5,000個
- リードタイム: 3 weeks
- B2B Value:
- Sustainability-focused hospitals
- Clinics seeking innovation
Summary Comparison Table
| メーカー | 国名 | 認証 | MOQ | リードタイム | OEMサービス |
|---|---|---|---|---|---|
| Polmed | Poland | ISO13485、CE | 10,000 | 2〜3週間 | はい |
| Rommedica | Romania | ID: CE、ISO 9001 | 5,000 | 3~4週間 | はい |
| MediEstetika | Bulgaria | ISO13485、CE | 3,000 | 2〜3週間 | はい |
| Medifem | Hungary | CE、ISO 13485 | 2,000 | 2 weeks | はい |
| Cytomed | Slovenia | ISO13485、CE | 5,000 | 3週間 | Yes (eco-friendly) |
5. Why Eastern Europe Is Becoming a Strategic Hub for Medical Consumables
Eastern Europe is rapidly emerging as a strategic hub for the production and export of medical consumables like disposable cytology brushes. This shift is being driven by a combination of favorable economic conditions, skilled labor, and proximity to major healthcare markets within the European Union.
Key Strategic Advantages
1. Cost-Effective Manufacturing
- 労働コスト: Eastern European countries offer significantly lower labor costs compared to Western Europe.
- Operational Overheads: Lower real estate and utility costs reduce overall production expenses.
- Pricing Advantage: Enables manufacturers to offer competitive B2B pricing for wholesale buyers.
2. EU Market Access
- Free Trade Zones: Manufacturers benefit from tariff-free trade within the EU.
- Harmonized Regulations: Products manufactured to EU MDR standards can be sold across all 27 EU member states.
- 迅速な配送: Central European location ensures quick delivery to hospitals, clinics, and labs.
3. Skilled Workforce
- Medical Device Engineering: Universities across the region produce highly skilled engineers and technicians.
- Multilingual Sales Teams: Facilitates international B2B communication and technical support.
4. Government Support
- Subsidies and Grants: Many governments provide funding to encourage medical device exports.
- Export Incentives: Encouragement for OEM partnerships and joint ventures with foreign companies.
5. Growing R&D Ecosystem
- University-Industry Collaboration: Many factories partner with medical faculties for innovation.
- Focus on Sustainability: Increasing number of manufacturers are developing eco-friendly and biodegradable cytology brushes.
6. Why Choose Jiangsu Hanheng as Your China-Based Cytology Brush Supplier
While Eastern Europe offers proximity to EU buyers, China remains a global powerhouse in manufacturing medical consumables. For buyers seeking reliable, high-volume sourcing with cutting-edge innovation, 江蘇漢恒医療技術有限公司 is the top choice in China.
About Jiangsu Hanheng
Founded in 2018, Jiangsu Hanheng is a leading manufacturer of medical testing consumables. The company operates a 10,000㎡ Class 100,000 cleanroom and exports globally with full regulatory certifications.
Why Global Buyers Trust Hanheng
| 特徴 | 江蘇漢恒の利点 |
|---|---|
| 規制遵守 | CE, ISO 13485, FDA, multiple utility model patents |
| 海外のサプライヤーは、多くの場合、カスタムブランディング、パッケージング、およびラベリングを備えた完全なOEMサービスを提供しています | Advanced facilities, automated production lines |
| 研究開発イノベーション | Dedicated team for product optimization and development |
| 製品範囲 | Full gynecological sampling portfolio including cytology brushes |
| カスタマイズとOEMサービス | Full private label capabilities for global distributors |
| より良いサンプル保存のための統合輸送培地 | Fast, reliable, and cost-efficient international shipping |
製品のハイライト
- Sterile Disposable Cytology Brushes: Designed for accurate cervical sample collection with soft-tip bristles and ergonomic handles.
- 子宮頸部サンプルコレクター: Compatible with various diagnostic testing systems.
- 婦人科検査キット: Complete kits for clinics and hospitals.
B2Bサービス
- OEM/ODM manufacturing with custom packaging
- Bulk pricing tiers for wholesale distributors
- Technical documentation and regulatory support
- Dedicated sales team fluent in English and experienced in global B2B
Ideal for
- International Medical Distributors
- Cervical Cancer Screening Program Suppliers
- Laboratory Product Resellers
- Hospital Procurement Officers
📩 To inquire about wholesale cytology brushes or request samples, contact Jiangsu Hanheng at [email protected] or visit the official website at www.hanheng-medical.com
7. How to Source Wholesale Cytology Brushes from Eastern Europe and China
Whether you’re a distributor, hospital procurement officer, or private label brand, sourcing disposable cytology brushes at wholesale prices requires a strategic approach. With Eastern Europe and China being two of the most prominent regions for medical consumables manufacturing, understanding the sourcing process can help streamline procurement and ensure quality, compliance, and reliability.
Step-by-Step Guide to Sourcing Wholesale Cytology Brushes
ステップ1:要件を定義する
Before reaching out to manufacturers, clearly define:
- 製品仕様: Bristle type, brush head size, shaft length, sterilization method
- 必要な認証: CE, FDA, ISO 13485 depending on your market
- パッケージングの好み: Individual sterile packs, bulk options, private label
- MOQ and Volume: Define your initial order quantity and monthly/quarterly volumes
- Delivery Deadlines: Plan lead times, especially for public tenders or clinical trials
以下のチェックリストを使用して、最適なサプライヤーを評価し、ショートリストします。
Use B2B platforms and resources to identify compliant, experienced suppliers:
| Platform/Resource | 説明 |
|---|---|
| Medica Trade Fair | Global medical device exhibition to meet suppliers |
| Alibaba / Made-in-China | B2B platforms with verified manufacturers |
| EU Business Directories | Manufacturer listings by certification and product |
| Hanheng Official Website | Direct contact for samples and quotes: www.hanheng-medical.com |
| Distributor Networks | Ask existing B2B contacts for referrals |
Step 3: Request Samples and Documents
Contact shortlisted manufacturers to request:
- 製品サンプル
- Quality and sterilization certificates
- CE, ISO, FDA documentation
- Factory audit reports (if available)
- OEM/ODM capability brochures
Step 4: Evaluate and Compare
Create a comparison matrix to evaluate suppliers based on:
| 基準 | Weight (%) | 備考 |
|---|---|---|
| 製品の品質 | 30% | Sample performance, design, sterility |
| 認証とコンプライアンス | 20% | CE/FDA for market entry |
| Cost Per Unit | 15% | Consider total landed cost |
| リードタイム | 10% | Days from order to delivery |
| OEM/Private Label Capabilities | 10% | カスタマイズオプション |
| Communication & Service | 10% | Responsiveness, professionalism |
| ロジスティクスサポート | 5% | Incoterms, shipping experience |
Step 5: Negotiate Terms and Place Trial Order
Once a supplier is selected, negotiate:
- Payment terms (usually 30% deposit, 70% before shipping)
- Delivery incoterms (EXW, FOB, DDP, etc.)
- カスタムブランディングと包装オプション
- Quality assurance processes
- アフターサービス
Start with a trial order (e.g., 5,000–10,000 units) to verify consistency before scaling up.
Sourcing from Eastern Europe vs. China: A Comparison
| ファクター | 東ヨーロッパ | 中国(例:Hanheng) |
|---|---|---|
| Proximity to EU | Close proximity, lower freight for EU buyers | Longer shipping times for EU, faster for Asia/Africa |
| 規制遵守 | EU MDR-compliant factories | Offers CE, FDA, ISO 13485, globally accepted |
| Manufacturing Costs | 中程度から高い | Lower manufacturing and labor costs |
| イノベーションと研究開発 | Moderate innovation | Strong R&D, especially Hanheng in cytology solutions |
| OEM/プライベートラベルサービス | Available, more limited | Comprehensive, scalable, and customizable |
| スケーラビリティ | Limited production capacity in some factories | High-volume production with automated lines |
| Minimum Order Quantities | Lower in some cases | Moderate, but flexible (Hanheng offers low MOQs) |
| リードタイム | 2~4週間 | 3–5 weeks (air) or 30–40 days (sea freight) |
パンデミック後の診断文化
- Use Incoterms Wisely: Negotiate EXW or FOB terms depending on your logistics network.
- Plan Inventory Cycles: Place staggered purchase orders to maintain steady supply.
- Audit Suppliers: If possible, conduct third-party audits or video inspections.
- Leverage Certifications: Use CE/FDA documentation to ease customs and compliance.
- Build Relationships: Strong supplier relationships lead to better credit terms and priority production.

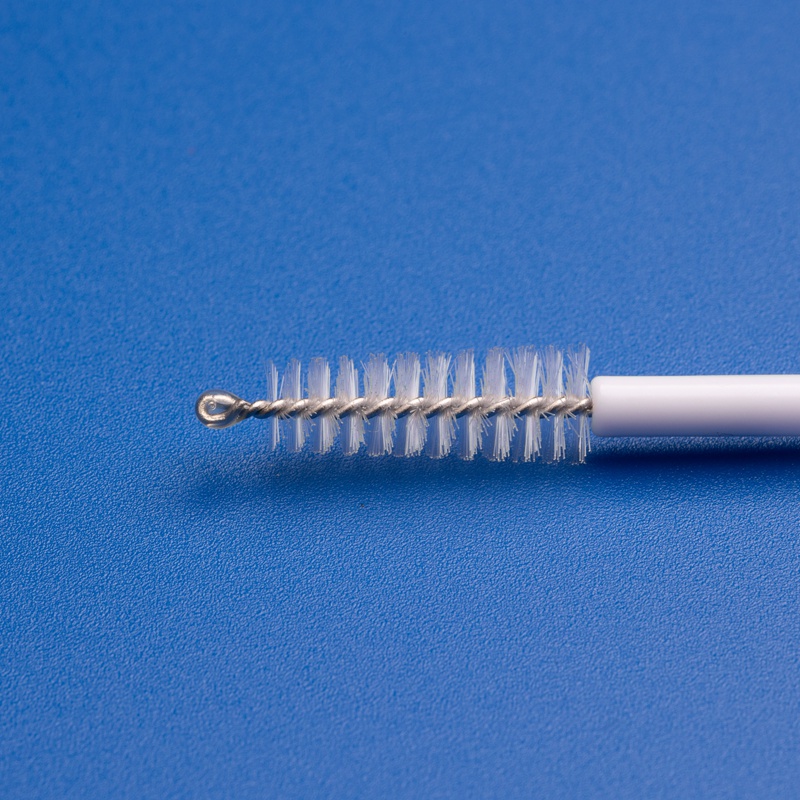

8. Frequently Asked Questions About Buying Bulk Cytology Brushes
Below are answers to common questions asked by wholesale buyers, procurement agents, and medical distributors sourcing disposable cytology brushes.
Q1: What certifications should I look for in a cytology brush supplier?
A: The most important certifications include:
- ISO 13485: Quality Management System for medical devices
- CEマーク: Required for distribution within the European Union
- FDA登録: Required for the U.S. market
- Sterilization Validation Reports: EO or Gamma sterilization validation
Hanheng, for example, holds CE, FDA, ISO 13485, and several utility model patents.
Q2: What is the typical MOQ for wholesale cytology brush orders?
A: Minimum Order Quantities vary by region and manufacturer:
- 東ヨーロッパ: 2,000–5,000 pieces
- 中国(漢恒): Flexible MOQs starting from 5,000 units, scalable to 100,000+
Q3: Can I brand the cytology brushes with my clinic or distributor logo?
A: Yes. Most manufacturers, including Jiangsu Hanheng, offer:
- OEMおよびODMサービス
- カスタムパッケージングとラベリング
- 多言語の説明書とIFU
- Branding design assistance
Q4: How long does it take to receive orders?
A: Lead times depend on order volume and shipping method:
| 地域 | Production Time | Shipping Time (Air) | Shipping Time (Sea) |
|---|---|---|---|
| 東ヨーロッパ | 2~4週間 | 2–5 days (within EU) | N/A |
| 中国(漢恒) | 3~5週間 | 5~10日 | 30–40 days |
Q5: Are cytology brushes single-use or reusable?
A: All cytology brushes discussed here are sterile, single-use devices designed for one-time use to prevent cross-contamination and ensure patient safety.
Q6: What packaging options are available?
A: Common packaging formats include:
- Individually sterile packed (blister or flow wrap)
- Bulk-packed (for institutions with sterile field capabilities)
- OEM kits (including brush, spatula, and specimen container)
Q7:バルク注文の前にサンプルを入手できますか?
A: Yes. Most manufacturers, including Hanheng, offer free samples for evaluation. Contact [email protected] をリクエストしてください。
7. 子宮頸部細胞診検査キットを大量に調達する方法:ステップバイステップガイド
As global demand for cervical cancer screening and gynecological diagnostics continues to grow, the need for high-quality, compliant, and cost-effective disposable cytology brushes is more pressing than ever. Eastern Europe offers proximity and regulatory alignment for EU buyers, while China—led by industry pioneers like 江蘇漢恒—delivers innovation, scale, and customization at unmatched value.
Why Jiangsu Hanheng Is the Trusted Choice for Global Buyers
- ✅ ISO 13485, CE, FDA Certified
- ✅ Full Suite of Gynecological Sampling Products
- ✅ Scalable OEM and Private Label Services
- ✅ World-Class Cleanroom Facilities
- ✅ Fast Global Shipping and Multilingual Support
Whether you’re a medical distributor, hospital procurement manager, or e-commerce B2B supplier, 江蘇漢恒 offers the reliability, performance, and support you need to grow your business in the women’s health diagnostics space.
📞 Ready to source high-quality cytology brushes at competitive wholesale prices?
👉 www.hanheng-medical.com
📧 連絡先: [email protected]
Let Hanheng help you deliver precision, safety, and value to your customers—worldwide.

江蘇漢恒医療技術有限公司
当社は、精密性、安全性、グローバルコンプライアンスを追求する、高品質な医療用消耗品のトップメーカーです。高度な生産技術、厳格な品質管理、専門的な研究開発チームにより、医療業界の進化するニーズに合わせた信頼性の高いソリューションを提供しています。



