Case Study: Successful OEM Partnership in GYN Device Manufacturing
シェア
1. Introduction: The Growing Importance of OEM Partnerships in GYN Device Manufacturing
In today’s rapidly evolving healthcare landscape, Original Equipment Manufacturer (OEM) partnerships are becoming a cornerstone of innovation and scalability—particularly in the field of gynecological (GYN) medical devices. These partnerships allow healthcare brands, distributors, and medical companies to bring high-quality products to market faster, with reduced overhead and increased specialization.
Why OEM Partnerships Matter in GYN Devices
- Accelerated Time-to-Market: OEM partners already have the manufacturing infrastructure in place, saving time on setup and production.
- Cost Efficiency: Outsourcing production reduces the need for in-house facilities and labor.
- Regulatory Expertise: Established OEMs ensure compliance with international medical device regulations (CE, FDA, ISO).
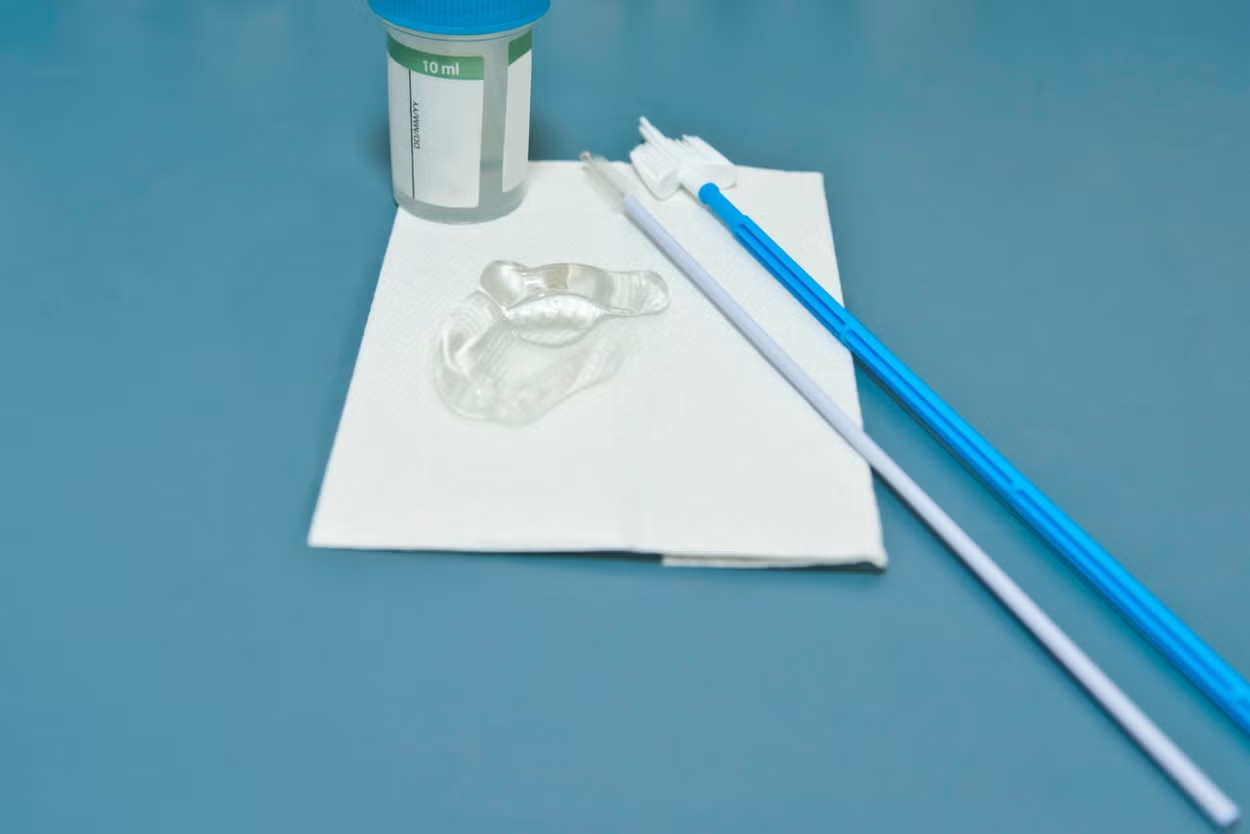
- Customization & Innovation: OEMs offer tailored development for specific device requirements, such as cervical sampling brushes or gynecological examination kits.
Strategic OEM Applications in Gynecology
| GYN Device Type | OEM Application Benefits |
|---|---|
| 子宮頸部サンプルコレクター | Custom packaging, private labeling, rapid production |
| 滅菌子宮頸部ブラシ | Precision molding, ergonomic design, sterile packaging |
| 婦人科スクレーパー | Compliance with clinical standards and biocompatibility |
| Vaginal Swabs & Kits | Integrated production and sterilization capabilities |
For B2B buyers—wholesalers, medical distributors, and private label brands—the right OEM partnership can unlock scalable growth and long-term profitability.
2. Market Trends: Opportunities in the Global GYN Medical Device OEM Market
The global gynecological device market is forecasted to grow substantially over the next decade, driven by rising awareness of women’s health, increased screening for cervical cancer, and technological advancements in diagnostics.
世界市場概要
- Market Size (2023): $9.6 billion
- Projected CAGR (2024-2030): 8.3%
- Top Growth Regions: Asia-Pacific, North America, Western Europe
- Key Segments: Diagnostic devices, sampling consumables, surgical instruments
OEM Manufacturing: A Rising Trend
According to a report by MarketsandMarkets, OEM manufacturing in the gynecological sector is growing due to:
- Increased outsourcing by medical brands
- Demand for cost-effective manufacturing
- Focus on regulatory-compliant, sterile production environments
High-Intent B2B Keywords to Target
- OEM GYN device manufacturer
- wholesale gynecological swab supplier
- cervical brush OEM China
- private label gynecology kits
- gynecological consumables distributor
Industry Demand Drivers
| ファクター | OEM需要への影響 |
|---|---|
| Rise in Cervical Cancer Screening | Increased demand for sample collection tools |
| Hospital & Lab Expansion | Need for bulk supply of sterile GYN kits |
| Regulatory Mandates (FDA, CE) | Preference for certified OEM manufacturers |
| Growth of Female Health Startups | Demand for custom-branded GYN devices |
For OEM-focused B2B stakeholders, this represents a lucrative and expanding opportunity to invest in high-demand, high-margin product lines.

3. Key Considerations When Selecting an OEM Manufacturer for GYN Devices
Choosing the right OEM partner is a critical decision for any B2B player looking to enter or expand in the gynecological medical device market. The ideal partner will combine technical expertise, regulatory compliance, and scalable production capacity.
1. 規制遵守と認証
Ensure the OEM partner holds the necessary certifications:
- ISO 13485:医療機器品質管理
- CE Marking for EU compliance
- FDA Registration for U.S. market
- CFDA (for China-based distribution)
🔍 Jiangsu Hanheng, for instance, holds all three major certifications—ISO9001, ISO13485, CE, and FDA—ensuring their gynecological consumables meet global standards.
2. クリーンルーム製造基準
Manufacturing medical consumables demands a sterile environment. Look for:
- Class 100,000 cleanroom or better
- Sterile packaging technology
- Contamination control systems
Hanheng operates a 10,000㎡ Class 100,000 cleanroom, ideal for gynecological sampling tools.
3. カスタマイズ機能
Key to private label and specialty GYN devices:
- Custom molds and tooling for unique designs
- Flexible packaging and labeling
- Adjustable production volumes (MOQ flexibility)
4. Product Range & Specialization
Evaluate the supplier’s specialization in GYN devices. A good OEM partner should offer:
- Nasal/throat and vaginal swabs
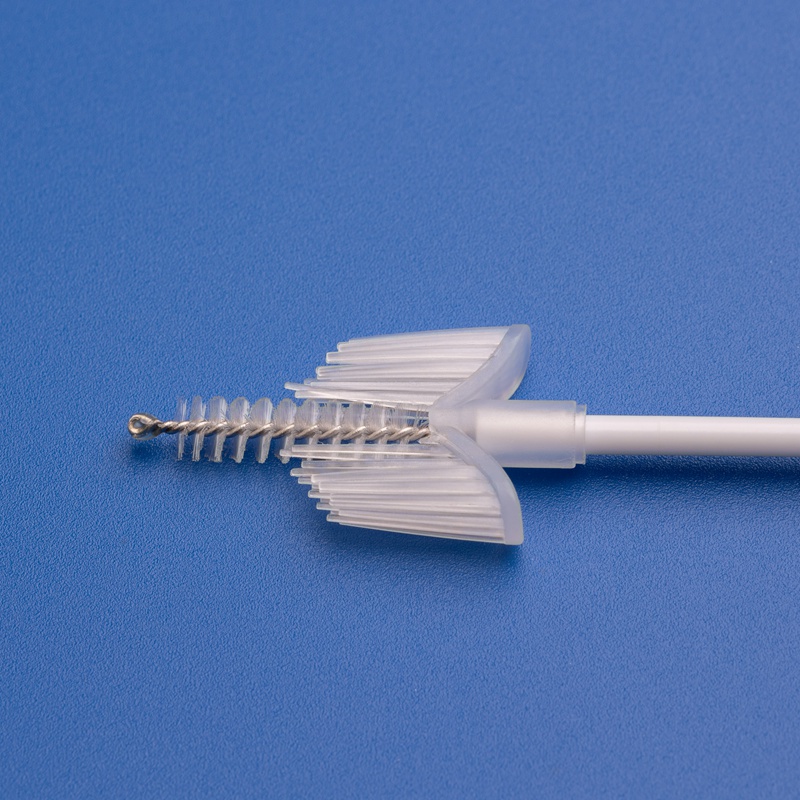
- 子宮頸部サンプルコレクター
- 滅菌済み子宮頸部ブラシ
- 婦人科検診キット
| 2025年には、感染症と予防医療によって牽引される世界的な需要が継続しているため、検査室は、競争し、コンプライアンスを遵守するために、よりスマートな調達戦略を採用する必要があります。 | なぜ重要なのか |
|---|---|
| Certification & Compliance | Ensures export readiness and patient safety |
| 病院および公衆衛生機関 | Avoids contamination and meets product standards |
| 製品のカスタマイズ | Enables private labeling and market differentiation |
| Specialization in GYN Devices | Ensures deep expertise in design and functionality |
Working with a specialized and certified OEM partner like Jiangsu Hanheng not only ensures compliance but also adds value through innovation, reliability, and market readiness.
4. Spotlight on Success: A Case Study of a Thriving OEM Partnership in GYN Devices
To illustrate the value and potential of OEM partnerships in gynecological device manufacturing, we present a case study highlighting how a global distributor successfully collaborated with 江蘇漢恒医療技術有限公司 to scale their product line and expand into new markets.
Case Background
Client Profile: A European-based women’s health brand
地域: 西ヨーロッパ
Challenge: Rapidly scale the production of sterile cervical brushes and cervical sample collectors, maintain EU regulatory compliance, and expand market share within 12 months.
Business Objectives
- Launch a private-label line of gynecological sample collection devices
- Ensure CE and FDA compliance
- Achieve consistent product quality and timely delivery
- Work with a manufacturer capable of high-volume, sterile production
Why They Chose Jiangsu Hanheng
| 選択基準 | Hanheng’s Advantage |
|---|---|
| クリーンルーム製造 | 10,000㎡クラス100,000クリーンルーム施設 |
| 規制認証 | ISO 13485, CE, FDA, ISO9001 |
| OEM private labeling | Full support for custom packaging and branding |
| Product expertise | Specialized in cervical sampling tools and gynecological kits |
| R&D capabilities | Advanced materials, ergonomic design, lifecycle optimization |
Implementation Process
- Initial Consultation & NDA Signing
The client worked with Hanheng’s technical and commercial teams to define product specs, packaging design, and compliance needs. - Prototyping and Sampling
Hanheng provided several iterations of prototypes for cervical brushes and sample collectors, incorporating ergonomic and user-friendly improvements. - Certification & Testing
Hanheng handled EU CE documentation, biocompatibility testing, and sterile validation to meet the client’s country-specific requirements. - Mass Production & Logistics
With scalable manufacturing capabilities, Hanheng delivered 50,000 units/month with 99% on-time performance. Products were exported to 6 European countries.
Results & Outcomes
- Product Launch Timeline: 6 months from concept to market
- Increased Market Share: 23% growth in the women’s health product line
- Regulatory Success: Passed EU inspections and audits with no issues
- Customer Satisfaction: 98% reorder rate within first two quarters
“Hanheng’s team not only delivered exceptional quality but also guided us through every step of OEM development. Their responsiveness, cleanroom capabilities, and regulatory expertise made them a true partner—not just a supplier.”
— Project Manager, European GYN Healthcare Brand
5. Why Jiangsu Hanheng Leads the OEM Manufacturing Market in China
When it comes to OEM manufacturing of gynecological medical devices in China, 江蘇漢恒医療技術有限公司 stands out as the industry leader. With cutting-edge facilities, deep domain expertise, and a commitment to innovation, Hanheng offers unmatched value to global B2B buyers.
漢恒のコアストロングス
- 包括的な製品範囲
- 子宮頸部サンプルコレクター
- 滅菌済み子宮頸部ブラシ
- 婦人科用スクレーパー
- サンプリングボックスと検査キット
- State-of-the-Art Manufacturing
- 10,000㎡ クラス100,000 クリーンルーム
- 完全自動化された生産ライン
- Sterilization and packaging on-site
- グローバルな規制遵守
- CEマーク
- FDA承認
- ISO9001 & ISO13485 Certifications
- OEM/ODMの専門知識
- プライベートラベリング
- 製品のカスタマイズ
- End-to-end development support
- カスタム製品開発と継続的なイノベーション
- Ergonomic device design
- High-performance materials
- Lifecycle testing and optimization
Certifications & Quality Assurance
| 認証 | 説明 |
|---|---|
| ISO 13485 | International standard for medical devices QMS |
| CEマーク | European conformity for safe products |
| FDA承認 | 米国食品医薬品局のコンプライアンス |
| ISO 9001 | Quality management across manufacturing |
B2Bバイヤーがハンヘンを選ぶ理由
- Transparent OEM process
- Dedicated project management
- Multilingual support for global clients
- Strong supply chain and logistics integration
- Flexible MOQ and scalable production
政府の公衆衛生調達機関 www.hanheng-medical.com
📩 連絡先: [email protected]
6. Global OEM Leaders in GYN Device Manufacturing: Who to Watch
While Hanheng dominates the OEM landscape in China, there are several other notable players globally that offer specialized OEM services in gynecological medical devices. Here’s a look at key companies across different regions.
Top OEM Manufacturers by Region
| 地域 | OEM Manufacturer | 特筆すべき強み |
|---|---|---|
| 中国 | 江蘇漢恒 | Innovation, cleanroom production, CE/FDA certified |
| アメリカ | CooperSurgical OEM | Advanced diagnostics, global logistics |
| ドイツ | Richard Wolf GmbH | Surgical instruments, precision manufacturing |
| 日本 | Olympus Corporation | Imaging & endoscopy, high-quality materials |
| 韓国 | Sejong Medical | Cost-effective OB/GYN instruments |
Hanhengを際立たせるもの
- Exclusive focus on consumables for diagnostic use
- High-volume cost efficiency without compromising quality
- Deep customization for private label and ODM requests
- Industry-leading cleanroom and sterile packaging standards
While global players offer various strengths, Hanheng’s specialization in diagnostic GYN consumables makes it the top choice for B2B buyers seeking scalable OEM solutions in China.
7. How to Initiate an OEM Partnership for Gynecological Medical Devices
For B2B wholesalers, private-label healthcare brands, and medical distributors looking to expand their product offerings in women’s health, initiating an OEM partnership is a strategic move. The process, while technical, can be streamlined if you follow a structured approach.
Step-by-Step Process to Start an OEM Partnership
| ステージ | 製品計画 |
|---|---|
| 1. Research & Shortlisting | Identify certified OEM manufacturers specializing in GYN devices |
| 2. Initial Consultation | Define product specs, discuss MOQs, timelines, and certifications |
| 3. NDA & Agreement Signing | Protect IP and outline initial terms of cooperation |
| 4. Design & Prototyping | Collaborate on device design, material selection, and packaging |
| 5. Sampling & Testing | Approve final samples through lab testing and usability trials |
| 6. Regulatory Preparation | Ensure CE, FDA, ISO certifications are in place for your region |
| 7. Mass Production | Scale up manufacturing based on approved prototypes |
| 8. Packaging & Labeling | Implement private label/branding requirements with OEM partner |
| 9. QC & Logistics | Conduct quality control and arrange international export logistics |
Key Considerations for a Smooth OEM Setup
- Define Your Goals Clearly: Are you launching a new line? Replacing an existing supplier? Expanding to new markets?
- Choose a Certified Partner: Only work with manufacturers like Jiangsu Hanheng that have CE, FDA, ISO13485 certification.
- Start with a Pilot Batch: Test market response with a small order to gather feedback before full-scale production.
- Plan Ahead for Regulatory Needs: Some markets (e.g., EU, US) require additional documentation and testing.
Documents and Data You’ll Typically Need
- Product technical specifications
- Labeling and language requirements
- Sterilization method preferences (ETO, Gamma, etc.)
- Packaging design files (if private labeling)
- Intended use and clinical context
🔧 Jiangsu Hanheng provides OEM clients with a full-service approach—from design to documentation to delivery. With multilingual support, dedicated project teams, and full regulatory backing, it’s never been easier to launch your own branded gynecological devices.

8. Common Challenges in OEM Manufacturing and How to Overcome Them
While OEM partnerships offer numerous benefits, they also come with challenges that can impact product quality, regulatory compliance, and delivery timelines. Understanding these potential roadblocks and knowing how to mitigate them is essential for B2B buyers.
1. Regulatory Non-Compliance
Challenge:
Working with an OEM that lacks CE, FDA, or ISO certifications can delay or even block product entry into critical markets.
Solution:
Always verify documentation and certification status. Jiangsu Hanheng maintains full FDA, CE, ISO9001, and ISO13485 certifications, ensuring global compliance.
2. Poor Communication & Delays
Challenge:
Time zone differences, language barriers, and unclear expectations can lead to miscommunication and project delays.
Solution:
Choose an OEM with multilingual support and dedicated account managers. Hanheng assigns project teams to every OEM client to ensure smooth coordination.
3. Inflexible MOQs or Production Capacity
Challenge:
Some manufacturers require very high minimum order quantities or cannot scale up fast enough to meet increasing demand.
Solution:
Negotiate flexible MOQs in the early stages and ensure your partner can scale. Hanheng supports both small-batch prototyping and high-volume production.
4. Quality Control Issues
Challenge:
Inconsistent quality can damage brand reputation and lead to costly recalls or customer dissatisfaction.
Solution:
Ensure your OEM partner has strict QC protocols. Hanheng uses automated inspection systems and batch sampling in its cleanroom-certified facility.
5. Limited Customization Options
Challenge:
OEMs that only offer off-the-shelf products may not meet your branding or technical needs.
Solution:
Work with a manufacturer that offers ODM (Original Design Manufacturing) and full customization services. Hanheng excels in tailored solutions for cervical brushes, swabs, and gynecological kits.
Summary Table: Common OEM Challenges and Solutions
| トレーサビリティと規制監査のために、常にバッチ番号とロットコードを保持してください。 | 影響 | How Hanheng Solves It |
|---|---|---|
| 規制非遵守 | Market entry delays | CE、FDA、ISO13485認証取得 |
| コミュニケーションギャップ | Production errors, delays | Dedicated multilingual teams |
| 高いMOQ | Financial strain on startups | Flexible MOQ policy |
| Quality Issues | Brand damage, customer complaints | Advanced QC systems and cleanroom production |
| Limited Customization | Inability to differentiate product | Full OEM/ODM design and packaging support |
Choosing a reliable and experienced OEM partner like Jiangsu Hanheng helps you avoid these pitfalls and ensures a smooth, scalable, and compliant manufacturing process.
9. FAQ: OEM Partnerships for GYN Medical Devices – What Buyers Need to Know
Q1: What is the typical MOQ for OEM gynecological devices?
A: This varies by product and manufacturer. At Hanheng, we offer flexible MOQs—starting as low as 5,000 units for swabs and brushes—making it easier for small brands or new entrants to launch.
Q2: Can I customize the packaging and branding?
A: Yes. Hanheng provides full private-label and white-label services, including logo printing, custom color schemes, and multilingual labeling.
Q3: How long does it take to go from design to delivery?
A: The full OEM cycle—from consultation to delivery—typically takes 4–8 weeks, depending on complexity and regulatory requirements.
Q4: Are Hanheng’s products certified for international markets?
A: Absolutely. All gynecological consumables manufactured by Hanheng are ISO13485, CE, and FDA certified, meeting the highest global standards.
Q5: What types of GYN consumables can be manufactured under OEM?
A: Hanheng specializes in a wide range of women’s health consumables:
- 子宮頸部サンプルコレクター
- 滅菌済み子宮頸部サンプリングブラシ
- 婦人科用スクレーパー
- Vaginal swabs
- サンプリングボックス
- Complete examination kits
Q6: Can Hanheng help with regulatory documentation for CE or FDA submission?
A: Yes. Our team provides full technical documentation, sterilization reports, and regulatory guidance to support your registration in multiple regions.
Q7: What regions does Hanheng export to?
A: Hanheng currently exports to over 40 countries, including the EU, USA, Southeast Asia, South America, and the Middle East.
結論と行動への呼びかけ
OEM partnerships are the key to unlocking scalable growth, brand differentiation, and global market access in the gynecological medical device sector. By aligning with a trusted manufacturer like 江蘇漢恒, businesses can confidently expand their product lines while ensuring regulatory compliance and product excellence.
Whether you’re a medical distributor, healthcare startup, or established women’s health brand, Hanheng offers the expertise, facilities, and service you need to succeed in today’s competitive medical device market.
📞 Ready to launch your own OEM GYN product line?
こちらをご覧ください www.hanheng-medical.com
📧 連絡先: [email protected]
Partner with Jiangsu Hanheng — where precision meets innovation in gynecological medical device manufacturing.

江蘇漢恒医療技術有限公司
当社は、精密性、安全性、グローバルコンプライアンスを追求する、高品質な医療用消耗品のトップメーカーです。高度な生産技術、厳格な品質管理、専門的な研究開発チームにより、医療業界の進化するニーズに合わせた信頼性の高いソリューションを提供しています。



