Fabricant de kits de frottis Pap pour le système de santé brésilien
Partager
1. Introduction : Le rôle des kits de frottis Pap dans la santé des femmes au Brésil
Cancer du col de l'utérus est l'un des cancers les plus évitables mais les plus répandus chez les femmes au Brésil. Selon le ministère de la Santé du Brésil, il s'agit du troisième cancer le plus courant chez les femmes dans le pays, avec environ 17 000 nouveaux cas par an. Le test de frottis Pap (test de Papanicolaou) reste la méthode la plus efficace pour la détection précoce des anomalies cervicales, faisant des kits de frottis Pap un pilier de la stratégie de santé publique du Brésil.
Alors que le Système unique de santé brésilien (SUS) continue d'élargir les programmes de dépistage du cancer du col de l'utérus, la demande pour des kits de frottis Pap de haute qualité provenant de fabricants fiables a explosé. Les hôpitaux publics, les cliniques et les laboratoires privés augmentent leurs achats pour atteindre les objectifs nationaux de dépistage — ce qui en fait une catégorie de produits essentielle pour les distributeurs médicaux, les importateurs et les grossistes en produits de santé.
Pourquoi les kits de frottis Pap sont importants dans le système de santé brésilien
- Détection précoce du cancer du col de l'utérus – Les kits de frottis Pap aident à identifier les cellules précancéreuses ou cancéreuses dans le col de l'utérus avant l'apparition de symptômes.
- Partie des programmes nationaux de dépistage – Le SUS du Brésil offre des tests de frottis Pap gratuits aux femmes âgées de 25 à 64 ans, tous les trois ans.
- Soutient l'équité en santé des femmes – En fournissant un accès au dépistage dans les zones rurales et mal desservies, les kits de frottis Pap favorisent l'équité en matière de soins de santé.
- Précision diagnostique – Des kits de haute qualité assurent une collecte d'échantillons appropriée, réduisant les faux négatifs et améliorant les résultats diagnostiques.
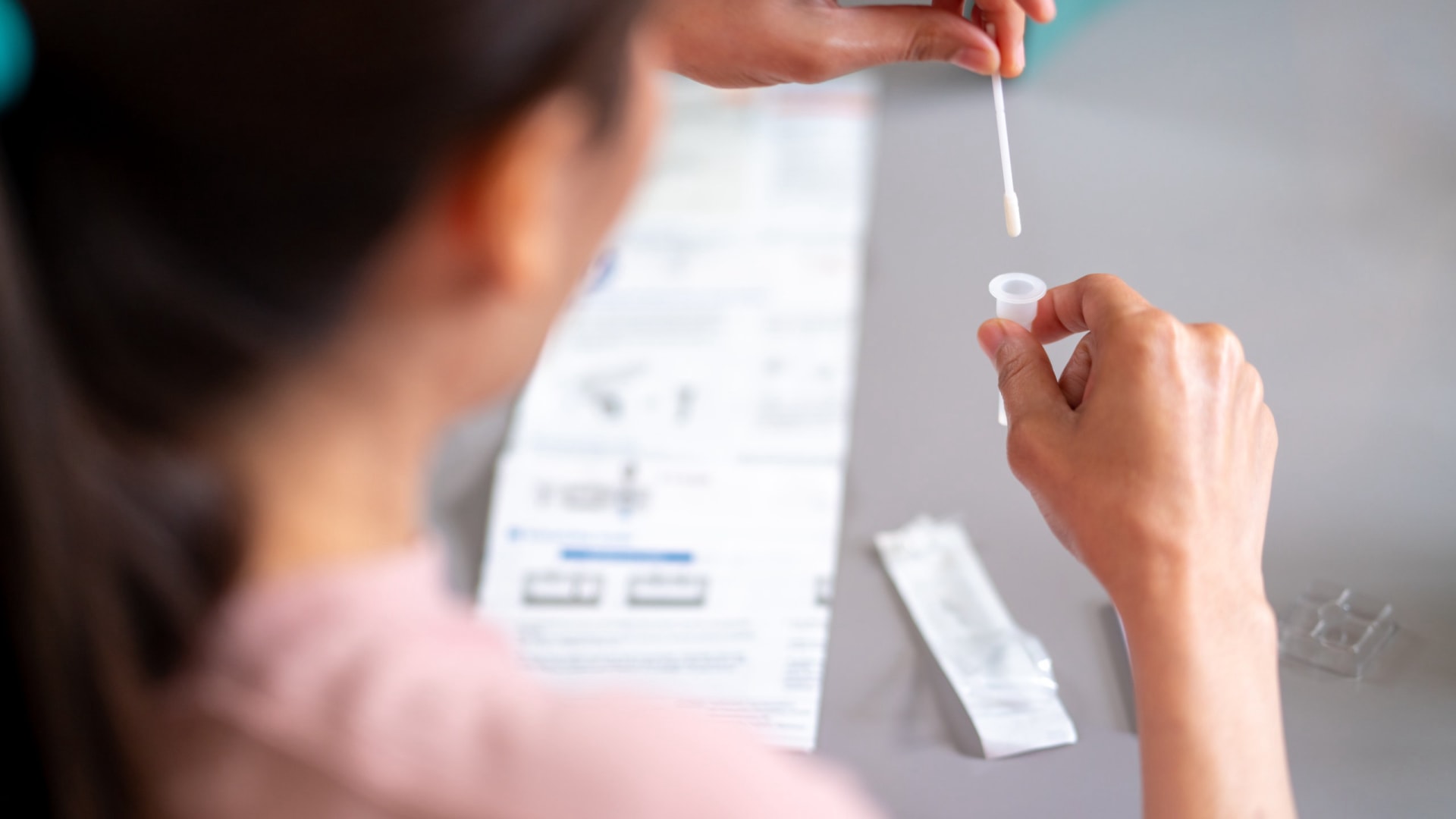
Composants d'un kit de frottis Pap standard
| Composant | Description | Objectif |
|---|---|---|
| Spéculum | Spéculum vaginal à usage unique ou réutilisable | Pour visualiser le col de l'utérus |
| Brosse cervicale | Brosse ou spatule stérile | Pour collecter les cellules cervicales |
| Milieu de transport | Flacon de cytologie liquide (LBC) ou lame | Préservation des cellules pour l'analyse en laboratoire |
| Mode d'emploi | Multilingue, avec des schémas clairs | Assure une collecte d'échantillons appropriée |
Les prestataires de soins de santé brésiliens, en particulier les organismes d'approvisionnement public et les réseaux de laboratoires privés, recherchent activement des fabricants fiables de kits de frottis Pap qui respectent les normes réglementaires, garantissent la cohérence des produits et offrent des options d'approvisionnement évolutives.
2. Tendances du marché : Croissance du dépistage et du diagnostic du cancer du col de l'utérus au Brésil
Le marché brésilien des diagnostics du cancer du col de l'utérus, y compris les kits de frottis Pap, connaît une transformation significative. L'augmentation des financements gouvernementaux, les campagnes de sensibilisation et les partenariats public-privé stimulent la demande pour des outils de dépistage précoce.
Principaux moteurs du marché
- Initiatives gouvernementales – Le « Plano Nacional de Prevenção e Controle do Câncer do Colo do Útero » du ministère de la Santé priorise le dépistage et le traitement précoce.
- Augmentation de la capacité des laboratoires – Expansion des laboratoires de cytologie et de pathologie dans les secteurs public et privé.
- Dépendance aux importations – Le Brésil importe une part importante de ses consommables médicaux, y compris les dispositifs de collecte de frottis Pap.
- Demande croissante du secteur privé – Le marché en expansion des assurances santé privées augmente la demande pour des outils diagnostiques premium.
Taille du marché et projections de croissance (2023–2028)
| Métrique | Estimation 2023 | Prévision 2028 | TCAC |
|---|---|---|---|
| Taille du marché des kits de frottis Pap (USD) | 42 millions de dollars | 68 millions de dollars | 10.2% |
| Nombre de dépistages | 21 millions | 32 millions | 8.7% |
| Dépendance aux importations | 65% | 60 % (prévu) | – |
Segments clés pour les distributeurs B2B
- Agences de santé publique (programmes d'approvisionnement SUS)
- Laboratoires de diagnostic privés (par ex., Fleury, Dasa)
- Réseaux hospitaliers (par ex., Rede D’Or São Luiz)
- Cliniques de santé des femmes
- Distributeurs en ligne d'équipements médicaux
Les importateurs et distributeurs de santé brésiliens cherchant à capitaliser sur cette croissance doivent travailler avec des fabricants certifiés ISO conformes aux normes mondiales, offrant une logistique fiable et des prix compétitifs.

.jpg)
3. Facteurs clés lors du choix d'un fournisseur de kits de frottis Pap pour le Brésil
Sélectionner un fabricant fiable de kits de frottis Pap est crucial pour assurer la qualité des produits, la sécurité des patients et la conformité réglementaire sur le marché médical strictement réglementé du Brésil. Voici les facteurs les plus importants pour les importateurs, les responsables des achats et les distributeurs en gros à considérer lors de l'approvisionnement en kits de frottis Pap :
1. Conformité réglementaire et certifications
- Enregistrement ANVISA – Obligatoire pour tous les dispositifs médicaux importés au Brésil.
- Certification ISO 13485 – Démontre l'adhésion aux systèmes de qualité des dispositifs médicaux internationaux.
- Marquage CE et approbation FDA – Indique une reconnaissance mondiale de la qualité, utile pour les équipes d'approvisionnement des hôpitaux et laboratoires.
2. Qualité des produits et normes de conception
- Assurance de la stérilité – Chaque composant doit être stérilisé et emballé individuellement.
- Conception ergonomique – Les brosses cervicales et spéculums doivent favoriser le confort et la facilité d'utilisation pour les cliniciens.
- Compatibilité – Les kits doivent être compatibles avec les flux de travail de cytologie sur lame et à base liquide.
3. Options de personnalisation et de marque
- Les distributeurs peuvent exiger :
- Étiquetage OEM/étiquetage privé
- Instructions en langue portugaise
- Kits personnalisés avec des composants spécifiques (p. ex., LBC uniquement, sans spéculum)
4. Capacités de fabrication
| Capacité | Importance | Notes |
|---|---|---|
| Production en salle blanche | Élevée | Empêche la contamination, essentiel pour la précision diagnostique |
| Fabrication à grande échelle | Moyen-élevé | Soutient les commandes B2B en gros volumes |
| Support R&D | Moyen | Capacité à innover ou à adapter les kits aux besoins locaux |
5. Logistique et soutien
- Délais de livraison – Délais standards de production et d'expédition.
- Documentation d'expédition – Doit respecter les exigences douanières brésiliennes et celles de l'ANVISA.
- Support après-vente – Matériel de formation, rappels de produits ou résolution des plaintes.
6. Tarification et conditions
- Échelles de tarification en gros
- Incoterms (FOB, CIF Santos, etc.)
- QCM (Quantités commandées minimales)
- Conditions de paiement (L/C, TT, etc.)
En évaluant ces critères, les acheteurs brésiliens peuvent sélectionner en toute confiance des fabricants qui non seulement respectent les normes techniques et réglementaires, mais agissent également comme des partenaires stratégiques pour le succès à long terme de la chaîne d'approvisionnement.
4. Top 5 fabricants de kits de frottis Pap pour le système de santé brésilien
La demande croissante du Brésil pour le dépistage du cancer du col de l'utérus a ouvert le marché aux fabricants nationaux et internationaux de kits de frottis cervico-vaginaux. Cependant, seul un groupe restreint d'entreprises répond de manière constante aux normes élevées de qualité des produits, de conformité réglementaire et de capacité de fabrication évolutive requises par les distributeurs brésiliens, les hôpitaux publics et les laboratoires de diagnostic.
Voici une comparaison des 5 principaux fabricants de kits de frottis cervico-vaginaux desservant le système de santé brésilien :
Aperçu des principaux fabricants
| Fabricant | Pays | Certifications | Points forts | Adapté au B2B |
|---|---|---|---|---|
| Jiangsu Hanheng Medical Technology Co, Ltd. | Chine | ISO 13485, CE, FDA | R&D innovante, production en salle blanche à haut volume, étiquetage OEM/privé | ✅ Oui |
| CooperSurgical | ÉTATS-UNIS | FDA, ISO 13485 | Qualité diagnostique premium, présence mondiale | ✅ Oui |
| Deltalab | Espagne | CE, ISO 9001, ISO 13485 | Large gamme de consommables de laboratoire | ✅ Oui |
| Hologic | ÉTATS-UNIS | FDA, CE | Solutions de cytologie à base liquide, systèmes automatisés | ⚠ Composants de kit limités uniquement |
| Kolplast | Brésil | ANVISA | Fabricant local, livraison rapide au Brésil | ✅ Oui |
Profils détaillés des fabricants
1. Jiangsu Hanheng Medical Technology Co, Ltd. (Chine)
Hanheng est le seul fabricant chinois que nous recommandons pour les kits de frottis cervico-vaginaux en raison de sa qualité de produit supérieure, de sa fabrication avancée et de sa conformité réglementaire. Fondée en 2018, Hanheng exploite une salle blanche de classe 100 000 de 10 000 m² et propose une gamme complète de dispositifs d'échantillonnage gynécologiques, incluant :
- Collecteurs d'échantillons cervicaux à usage unique
- Brosses cervicales stériles
- Grattoirs gynécologiques
- Boîtes d'échantillonnage
- Kits complets de frottis cervico-vaginaux avec options OEM/ODM
Pourquoi les distributeurs brésiliens choisissent Hanheng :
- Fabrication à grande échelle pour les commandes en vrac
- Certifié ISO 13485 et CE
- Soutien pour l'étiquetage en portugais et la documentation ANVISA
- Logistique d'exportation fiable vers le Brésil
- Tarification compétitive pour les acheteurs en gros
📌 Visitez : www.hanheng-medical.com
📧 Contact : [email protected]
2. CooperSurgical (États-Unis)
Leader mondial dans les diagnostics de la santé des femmes, CooperSurgical propose des dispositifs de collecte avancés et des outils de cytologie. Leur brosse Papette® est largement utilisée dans les laboratoires privés et les hôpitaux. Cependant, les prix de Cooper sont premium, les mieux adaptés aux fournisseurs de soins de santé privés haut de gamme au Brésil.
Points forts :
- Élevée précision diagnostique
- Approuvée par les grands laboratoires de diagnostic
- Performance clinique prouvée
Limites :
- Coût unitaire plus élevé
- Flexibilité limitée pour l'étiquetage privé
3. Deltalab (Espagne)
Deltalab propose une large gamme de consommables de laboratoire, y compris des brosses d'échantillonnage cervical et des lames de cytologie. Avec les certifications ISO et CE, ils constituent un choix solide pour les distributeurs à la recherche de produits fabriqués en Europe à un prix intermédiaire.
Points forts :
- Gamme de produits flexible
- Tarification modérée
- Documentation d'exportation fiable
Limites :
- Moins d'offres de kits complets
- Nécessite un assemblage local au Brésil
4. Hologic (États-Unis)
Hologic est le fabricant du ThinPrep® Pap Test, un système de cytologie liquide-based largement utilisé dans le monde entier. Bien que Hologic excelle dans les systèmes de dépistage automatisés, il fournit principalement les consommables et les flacons, et non des kits complets de frottis Pap.
Points forts :
- Standard d'or en technologie LBC
- Largement utilisé dans les grands laboratoires de diagnostic
Limites :
- Non adapté aux kits de frottis Pap manuels
- Coûteux et dépendant du système
5. Kolplast (Brésil)
Kolplast est un fabricant brésilien de produits gynécologiques et diagnostiques. Leurs kits de frottis Pap sont approuvés par l'ANVISA et rapidement accessibles aux cliniques et hôpitaux locaux.
Points forts :
- Fabriqué localement
- Livraison et service rapides
- Enregistré auprès de l'ANVISA
Limites :
- Innovation limitée
- Peut ne pas répondre aux normes de qualité internationales
5. Préférence croissante pour les fabricants internationaux par les distributeurs brésiliens
Bien que la production locale ait ses avantages, les distributeurs brésiliens de soins de santé se tournent de plus en plus vers les fabricants internationaux pour les kits de frottis Pap, en particulier en ce qui concerne la qualité, l'innovation et l'évolutivité.
Pourquoi les acheteurs brésiliens préfèrent les fournisseurs internationaux
1. Normes de qualité plus élevées
Les fabricants internationaux, en particulier les usines certifiées ISO 13485, dépassent souvent les normes de production locales. Cela se traduit par :
- Meilleure préservation des échantillons
- Amélioration de la précision diagnostique
- Confort accru pour les patients
2. Tarification compétitive à grande échelle
Grâce aux économies d'échelle et à l'automatisation avancée, les fabricants internationaux comme Hanheng peuvent offrir de meilleurs prix pour les commandes en gros, même après prise en compte des frais de transport et des droits d'importation.
3. Flexibilité pour l'étiquetage privé et l'OEM
De nombreux distributeurs brésiliens souhaitent commercialiser sous leur propre marque. Les fournisseurs internationaux, en particulier en Asie, sont plus ouverts à :
- Marque OEM
- Emballages personnalisés en portugais
- Modifications des composants des kits
4. Portefeuilles de produits plus larges
Les principaux fabricants internationaux proposent non seulement des kits de frottis Pap, mais aussi une gamme de consommables gynécologiques et diagnostiques connexes. Cela permet aux distributeurs de :
- Regrouper les produits
- Rationaliser les achats
- Offrir des solutions complètes aux cliniques et laboratoires
Tendances d'importation au Brésil (Kits de frottis Pap et gynécologie)
| Année | % de kits importés | Principaux pays sources d'importation |
|---|---|---|
| 2020 | 62% | Chine, États-Unis, Allemagne |
| 2021 | 65% | Chine, Espagne, États-Unis |
| 2022 | 67% | Chine, États-Unis, France |
Alors que cette tendance se poursuit, les distributeurs et responsables des achats médicaux brésiliens sont invités à évaluer les fournisseurs internationaux non seulement sur le prix, mais aussi sur leur capacité à se conformer aux réglementations ANVISA et à soutenir les exigences de documentation locale.
6. Pourquoi choisir Hanheng comme votre fabricant de confiance de kits de frottis Pap en Chine
Jiangsu Hanheng Medical Technology Co., Ltd.. devient rapidement le choix numéro un pour les acheteurs de kits de frottis Pap en Amérique latine, y compris au Brésil. L'entreprise offre un mélange d'innovation, de qualité et de service que peu de concurrents peuvent égaler.
Principaux avantages de travailler avec Hanheng
1. Gamme complète de produits
Hanheng fabrique tous les principaux composants utilisés dans les kits de frottis Pap :
- Brosse cervicale (stériles, conceptions ergonomiques)
- Collecteurs d'échantillons cervicaux à usage unique
- Spéculums (diverses tailles)
- Grattoirs gynécologiques et boîtes de prélèvement
Ces éléments peuvent être commandés individuellement ou regroupés en kits complets de frottis Pap, personnalisés selon les besoins de votre marché.
2. Fabrication avancée et contrôle qualité
- 10 000㎡ Salle blanche de classe 100 000
- Certifications ISO 13485, CE et FDA
- Lignes d'assemblage et d'emballage automatisées
- Traçabilité par lots et assurance de stérilité
3. Personnalisation pour le marché brésilien
- OEM/Étiquetage privé
- Instructions en langue portugaise
- Emballage des kits adapté aux protocoles ANVISA
- Soutien pour la documentation d'importation et l'enregistrement
4. Services d'exportation B2B dédiés
Hanheng fournit un soutien de bout en bout pour les importateurs brésiliens :
- Équipe d'exportation bilingue
- Délais de traitement rapides
- Devis CIF vers les ports brésiliens (par ex., Santos, Rio de Janeiro)
- Conseils réglementaires pour la soumission ANVISA
5. Réalisations prouvées
Hanheng fournit déjà les principaux réseaux hospitaliers, laboratoires de diagnostic et distributeurs dans :
- Amérique latine
- L'Europe
- Asie du Sud-Est
- Moyen-Orient
📧 Pour les acheteurs brésiliens, contactez : [email protected]
🌐 Site web : www.hanheng-medical.com
Que vous soyez un officier des achats gouvernementaux, un distributeur privé ou une chaîne de laboratoires de diagnostic, Hanheng fournit un approvisionnement fiable et évolutif en kits de frottis Pap adaptés au système de santé brésilien.
7. Comment importer des kits de frottis Pap au Brésil : Guide étape par étape pour l'achat en gros
L'importation de kits de frottis Pap au Brésil implique une série de procédures réglementaires et logistiques qui doivent être strictement suivies pour assurer la conformité avec l'ANVISA (Agência Nacional de Vigilância Sanitária) et les autorités douanières brésiliennes. Pour les distributeurs médicaux, les départements d'achats hospitaliers et les groupes de laboratoires privés, comprendre ce processus est essentiel pour éviter des retards coûteux et assurer une livraison ponctuelle aux prestataires de soins de santé.
Voici un guide complet et étape par étape adapté aux acheteurs B2B souhaitant importer des kits de frottis Pap au Brésil.
Étape 1 : Identifier un fabricant certifié
Commencez par sélectionner un fabricant répondant aux critères suivants :
- Certifié ISO 13485
- Conforme CE, FDA ou ANVISA (s'il est déjà enregistré au Brésil)
- Expérience dans l'exportation vers l'Amérique latine
- Capacité à fournir un étiquetage et une documentation en portugais
📌 Recommandé : Jiangsu Hanheng Medical Technology Co, Ltd.
🌐 www.hanheng-medical.com | 📧 [email protected]
Étape 2 : Vérifiez l'enregistrement du produit auprès de l'ANVISA
Tous les dispositifs médicaux et consommables, y compris les kits de frottis Pap et leurs composants (brosses, racloirs, etc.), doivent être enregistrés auprès de l'ANVISA avant l'importation.
Options :
- Utilisez un importateur avec un enregistrement ANVISA existant
Si votre importateur a déjà le produit enregistré, l'importation peut se dérouler rapidement. - Enregistrez le produit vous-même
Vous devrez peut-être soumettre un DCB (Dossiê de Cadastro de Produto) ou un DCM (Dossiê de Comunicação de Material) via une société brésilienne de réglementation ou directement via votre propre compte ANVISA.
Documents requis pour l'enregistrement ANVISA :
- Certificat de libre vente (CFS) du pays d'origine
- Certificats ISO 13485 et CE
- Dossier technique du produit
- Étiquettes de produit et IFU en portugais
- Certification GMP du fabricant (si applicable)
Étape 3 : Établissez un importateur légal (représentant local)
Pour importer des kits médicaux au Brésil, vous devez travailler avec une entreprise brésilienne enregistrée au CNPJ autorisée à agir en tant qu'importateur légal et responsable de la conformité réglementaire.
Options incluent :
- Votre propre filiale brésilienne
- Un partenaire de distribution local
- Un cabinet de conseil réglementaire agréé
Les responsabilités clés de l'importateur légal incluent :
- Soumission de la documentation à l'ANVISA
- Gestion du dédouanement
- Gestion des rappels de produits (si nécessaire)
Étape 4 : Préparez l'expédition internationale
Collaborez avec votre fabricant pour finaliser les conditions d'expédition. La plupart des importateurs choisissent :
- CIF (Coût, Assurance, Fret) vers le port de Santos ou Rio de Janeiro
- EXW ou FOB si vous utilisez un transitaire
📦 Emballage typique pour les kits de frottis Pap de Hanheng :
| Type d'emballage | Détails |
|---|---|
| Boîte intérieure | 25 kits par boîte |
| Carton maître | 10 boîtes intérieures (250 kits) |
| Palette | 20–40 cartons selon la quantité minimale de commande |
Assurez-vous que le fabricant fournit :
- Facture commerciale
- Liste de colisage
- Certificat d'origine
- Étiquettes d'expédition en langue portugaise (si requis)
- FDS (fiche de données de sécurité des matériaux) du produit
Étape 5 : Dédouanement et fiscalité
Les douanes brésiliennes (Receita Federal) exigent ce qui suit :
- Déclaration d'importation (DI)
- Facturation électronique (NF-e)
- Documents de licence ANVISA
- Classification sous le bon code NCM
Code NCM courant pour les kits de frottis Pap :
- 3822.00.90 – Réactifs de diagnostic ou de laboratoire sur un support, réactifs de diagnostic préparés
Taxes et droits :
| Type de taxe | Taux (environ) |
|---|---|
| II (Taxe d'importation) | 0–14 % |
| IPI (Taxe sur les produits industrialisés) | 0–15 % |
| PIS/COFINS | 9.65% |
| ICMS (Taxe d'État) | 7–18 % selon l'État |
Note : De nombreux dispositifs médicaux peuvent bénéficier d'exemptions fiscales dans le cadre des programmes d'accès à la santé du Brésil.
Étape 6 : Distribuez aux utilisateurs finaux
Une fois le dédouanement terminé, distribuez les kits de frottis Pap via votre réseau de :
- Hôpitaux publics (via appels d'offres gouvernementaux)
- Laboratoires de diagnostic privés (par ex., Dasa, Fleury)
- Cliniques de santé des femmes
- Plateformes B2B en ligne (Bionexo, MedeMarket)
Assurez-vous que tous les kits :
- Possèdent un étiquetage en portugais
- Incluent des IFU (instructions d'utilisation)
- Sont suivis par numéros de lots et dates d'expiration
Étape 7 : Maintenez la conformité et passez de nouvelles commandes
- Suivez les performances du produit et les retours des clients
- Surveillez les stocks et les dates d'expiration
- Repassez commande auprès de votre fournisseur à l'avance pour éviter les ruptures
- Maintenez la conformité aux exigences de surveillance post-marché de l'ANVISA
En établissant un processus d'importation répétable avec un fabricant fiable comme Hanheng, les distributeurs brésiliens peuvent assurer un approvisionnement constant, une conformité réglementaire et la satisfaction des clients.


8. Questions fréquemment posées sur l'achat en gros de kits de frottis Pap pour le Brésil
Pour aider les acheteurs B2B, les officiers des achats hospitaliers et les distributeurs médicaux à prendre des décisions d'achat éclairées, voici les réponses aux questions les plus courantes sur l'approvisionnement et l'importation de kits de frottis Pap au Brésil.
Q1 : Puis-je importer des kits de frottis Pap sans enregistrement ANVISA ?
Non. Tous les consommables médicaux utilisés pour le diagnostic humain doivent être enregistrés auprès de l'ANVISA avant l'importation. Vous pouvez soit enregistrer le produit vous-même, soit travailler avec un partenaire local qui a déjà le produit enregistré.
Q2 : Quelles sont les quantités minimum de commande (MoQ) pour les commandes en gros chez Hanheng ?
Hanheng fixe généralement les MoQ en fonction du type de produit :
- Brosses cervicales : 10 000 unités
- Kits complets : 5 000 kits
- Kits OEM personnalisés : Négociable, à partir de 3 000 kits
Des remises sur volume sont disponibles pour les commandes dépassant 50 000 unités.
Q3 : Les kits de frottis cervico-vaginal de Hanheng sont-ils compatibles avec les systèmes de cytologie en phase liquide (LBC) ?
Oui. Hanheng fabrique des brosses cervicales et des collecteurs entièrement compatibles avec les systèmes LBC couramment utilisés au Brésil, y compris ThinPrep et SurePath.
Q4 : Puis-je demander des instructions et un étiquetage en portugais ?
Oui. Hanheng prend en charge une personnalisation OEM complète, incluant :
- Instructions d'utilisation (IFU) en portugais
- Étiquetage avec la marque du distributeur
- Conception d'emballages personnalisés
Cela garantit la conformité aux réglementations ANVISA et aux lois brésiliennes de protection des consommateurs.
Q5 : Combien de temps faut-il pour recevoir une expédition en vrac au Brésil ?
Délais typiques :
- Production : 15 à 25 jours ouvrables (selon la taille de la commande)
- Expédition (fret maritime vers Santos/Rio) : 25–30 jours
- Dédouanement : 7–10 jours ouvrables
Délai total : Environ 45 à 60 jours de la confirmation de la commande à la livraison.
Q6 : Quelles certifications les kits de frottis cervico-vaginal de Hanheng possèdent-ils ?
- ISO 13485 : Gestion de la qualité des dispositifs médicaux
- CE : Conformité aux normes de sécurité européennes
- FDA : Conformité au marché américain
- Brevets de modèle d'utilité pour l'innovation de conception
- Éligibles à l'enregistrement ANVISA
Q7 : Puis-je commander des kits d'échantillon avant de passer une commande en vrac ?
Oui. Hanheng propose des kits d'échantillon pour évaluation. Ils sont généralement expédiés par courrier express (DHL/FedEx) et incluent divers types de brosses, racloirs et options d'emballage.
Q8 : À qui dois-je m'adresser pour obtenir un devis ou passer une commande ?
Vous pouvez contacter l'équipe d'exportation de Hanheng :
📧 Courriel : [email protected]
🌐 Site web : www.hanheng-medical.com
Indiquez votre quantité de commande estimée, la configuration du kit et le port de destination pour un devis CIF personnalisé.
9. Conclusion : Sécurisez votre chaîne d'approvisionnement en kits de frottis Pap en toute confiance
Alors que le Brésil continue d'élargir ses initiatives nationales de dépistage du cancer du col de l'utérus, la demande pour des kits de frottis cervico-vaginal de haute qualité et abordables n'a jamais été aussi forte. Les distributeurs, hôpitaux et professionnels des achats doivent s'associer à des fabricants capables de fournir de manière constante des produits conformes aux réglementations, cliniquement fiables et à grande échelle.
Jiangsu Hanheng Medical Technology Co, Ltd. se distingue comme le fournisseur principal pour les acheteurs B2B au Brésil à la recherche de :
✅ Fabrication certifiée (ISO 13485, CE, FDA)
✅ Production en salle blanche et R&D avancée
✅ Prise en charge complète pour l'étiquetage privé et l'OEM
✅ Documentation en portugais et kits prêts pour ANVISA
✅ Tarification en gros compétitive pour les grands volumes
Que vous soyez une agence gouvernementale approvisionnant des hôpitaux publics ou un distributeur médical privé développant votre portefeuille de diagnostics, Hanheng offre le mélange idéal de qualité, de service et de valeur.
📧 Contactez-nous dès aujourd'hui à [email protected]
🌐 Visitez www.hanheng-medical.com pour demander un devis ou un catalogue.
En choisissant le bon fabricant, vous ne faites pas seulement l'acquisition d'un produit — vous investissez dans la santé et le bien-être de millions de femmes brésiliennes. Faites le bon choix. Partenariez avec Hanheng.

Jiangsu Hanheng Medical Technology Co, Ltd.
Nous sommes l'un des principaux fabricants de consommables médicaux de haute qualité et nous nous engageons à assurer la précision, la sécurité et le respect des normes internationales. Grâce à une technologie de production avancée, à un contrôle de qualité strict et à une équipe de recherche et développement dévouée, nous fournissons des solutions fiables adaptées aux besoins changeants de l'industrie des soins de santé.



