Rationalisation de la production de kits avec des composants OEM pour frottis Pap

Partager
1. Introduction : L'importance des composants OEM pour frottis Pap dans la production de kits
Dans l'effort mondial de lutte contre cancer du col de l'utérus, les tests de frottis Pap jouent un rôle essentiel dans la détection précoce et la prévention. Alors que la demande de dépistage cervical efficace et précis augmente, le besoin de kits médicaux fiables et de haute qualité devient de plus en plus urgent. Une part importante de ces kits sont produits à l'aide de composants du fabricant d'équipement d'origine (OEM), des pièces spécialisées fabriquées par des fournisseurs tiers et intégrées dans des kits de diagnostic complets par des sociétés de dispositifs médicaux ou des laboratoires.
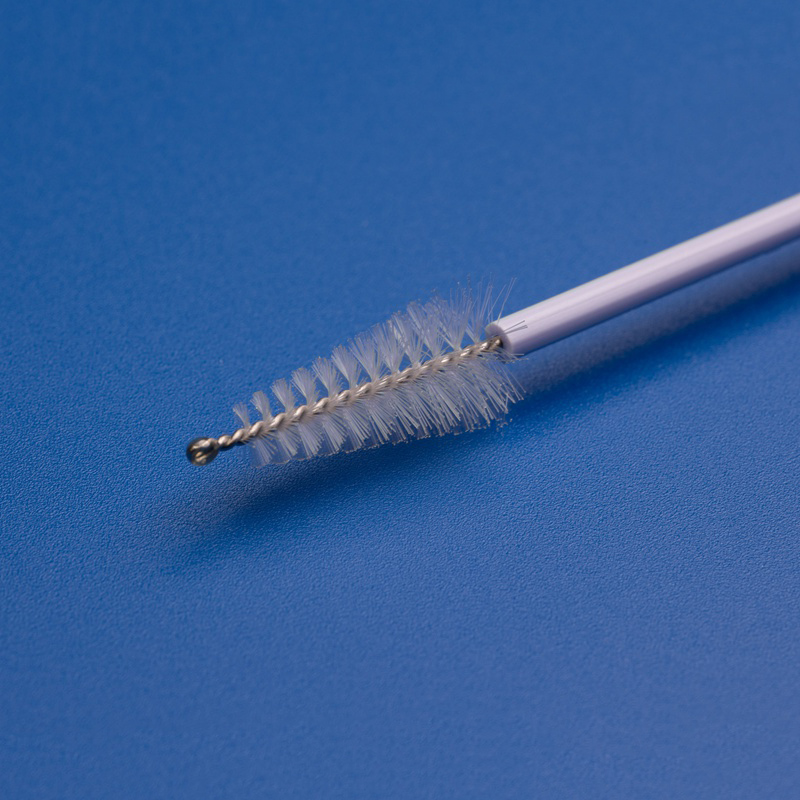
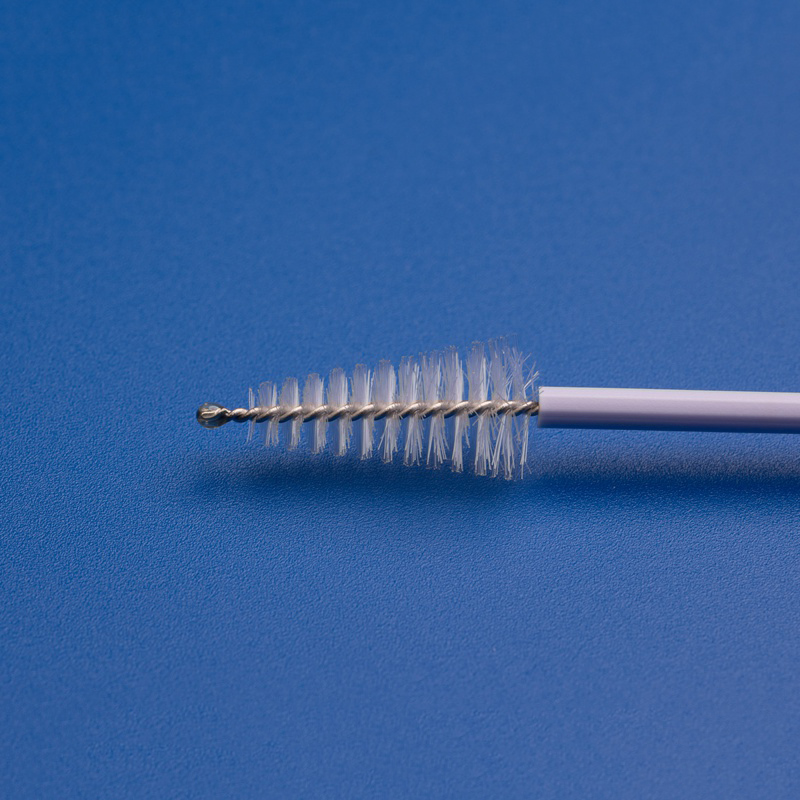
Les composants OEM pour frottis Pap, tels que les brosses cervicales, les spatules, les boîtes d'échantillonnage et les tubes de collecte, sont essentiels pour rationaliser les processus de production, garantir une qualité constante et répondre à la demande croissante d'outils de diagnostic gynécologique dans les systèmes de santé, les hôpitaux et les laboratoires.
Pourquoi les composants OEM sont importants dans la fabrication de kits médicaux
- Cohérence de la qualité: Les fournisseurs OEM se spécialisent dans la production de composants spécifiques à grande échelle, garantissant l'uniformité et le respect des normes de qualité médicale.
- Efficacité des coûts: L'externalisation de la fabrication de composants réduit les frais généraux de la production interne et permet des économies d'échelle.
- Accélération de la mise sur le marché: S'associer à un fournisseur OEM fiable accélère les délais d'assemblage et de distribution des kits.
- Conformité réglementaire: Les fournisseurs OEM bien établis garantissent que leurs produits sont conformes aux normes ISO, FDA et CE, ce qui est essentiel pour l'accès au marché international.
Composants clés d'un kit de frottis Pap typique
| Composant | Fonction | Options de personnalisation OEM |
|---|---|---|
| Brosse cervicale | Recueille des cellules cervicales pour la cytologie ou les tests HPV | Longueur de la tige, forme des poils |
| Spatule/grattoir de cytologie | Utilisé avec la brosse pour la collecte de cellules ectocervicales | Conception de la poignée, matériau |
| Tube/flacon de collecte | Contient l'échantillon pour le transport et les tests | Étiquetage, conservateurs |
| Boîte d'échantillonnage | Regroupe tous les articles pour un transport hygiénique | Image de marque, matériau d'emballage |
| Notice d'instructions | Guide les cliniciens ou les patients sur l'utilisation appropriée | Personnalisation multilingue |
En intégrant des composants OEM dans leur chaîne de fabrication, les producteurs de kits de diagnostic médical peuvent se concentrer sur la conception, l'image de marque et la distribution, tout en s'appuyant sur des fournisseurs de confiance pour des pièces haute performance qui répondent aux exigences cliniques.
2. Demande du marché et tendances de croissance des produits de dépistage du cancer du col de l'utérus
Le marché mondial du dépistage du cancer du col de l'utérus connaît une tendance à la hausse significative, stimulée par une sensibilisation accrue, des programmes de dépistage gouvernementaux et des avancées technologiques dans les outils de diagnostic. Les composants OEM pour frottis Pap sont directement liés à la croissance de ce marché, d'autant plus que de plus en plus de fabricants de kits de diagnostic cherchent à augmenter leur production, à pénétrer de nouveaux marchés et à assurer la conformité réglementaire.
Aperçu du marché du dépistage du cancer du col de l'utérus
- Taille du marché mondial (2023): Estimée à 8,5 milliards de dollars américains
- TCAC projeté (2024–2030): 6.2%
- Facteurs clés:
- Programmes de dépistage financés par le gouvernement
- Augmentation des taux de complications liées au VPH
- Demande croissante de kits de dépistage à domicile
- Expansion des laboratoires de diagnostic dans les marchés émergents
Aperçu de la demande régionale
| Région | Caractéristiques du marché | Niveau d'opportunité OEM |
|---|---|---|
| Amérique du Nord | Sensibilisation élevée au dépistage, conformité FDA requise | Élevée |
| L'Europe | Cadre réglementaire solide (CE), demande de kits durables | Élevée |
| Asie-Pacifique | Croissance rapide du marché, besoins non satisfaits importants dans les soins de santé en milieu rural | Très élevé |
| Amérique latine | Expansion des programmes de dépistage gynécologique | Moyen |
| Afrique | Marché en phase de démarrage, programmes de santé financés par des donateurs | Moyen |
Prévisions de la demande de composants OEM
- Brosses cervicales jetables: Demande croissante en raison de la préférence pour les composants stériles à usage unique
- Boîtes d'échantillonnage: Essentiel pour le transport et l'étiquetage ; forte demande dans les kits de test à domicile
- Spatules de cytologie: Toujours largement utilisées en combinaison avec des brosses pour une collecte complète d'échantillons
Tendances ayant un impact sur les fournisseurs OEM
- Demande de personnalisation: Les assembleurs de kits souhaitent des composants en marque blanche avec leur propre image de marque et leurs propres instructions.
- Durabilité: Les matériaux biodégradables ou recyclables deviennent une exigence dans certaines régions.
- Intégration numérique: Des codes QR et des étiquettes à code-barres pour le suivi des échantillons sont intégrés dans les emballages OEM.

3. Principales considérations lors du choix des fournisseurs OEM pour les composants de kits de frottis Pap
Pour les acheteurs B2B, y compris les fabricants de dispositifs de diagnostic, les réseaux de laboratoires et les distributeurs de soins de santé, la sélection du bon fournisseur OEM pour les composants de kits de frottis Pap est essentielle pour garantir la fiabilité, la conformité et l'évolutivité. Voici les principales considérations que les responsables des achats et les développeurs de produits doivent évaluer.
1. Certification et conformité
- Normes requises:
- ISO 13485 (Gestion de la qualité des dispositifs médicaux)
- ISO 9001 (Gestion générale de la qualité)
- Marquage CE (Conformité au marché européen)
- Enregistrement FDA (Accès au marché américain)
- Pourquoi c'est important: Ces certifications garantissent que les composants répondent aux normes internationales de sécurité et de performance, ce qui réduit les obstacles réglementaires lors de l'exportation de kits finis.
2. Capacités de fabrication
- Normes relatives aux salles blanches: Les salles blanches de classe 100 000 sont idéales pour la fabrication de composants stériles et sans contamination.
- Évolutivité de la production: Les fournisseurs doivent être capables de répondre aux petites et grandes commandes sans délai.
- Capacités de R&D: Les principaux OEM fournissent une assistance à la conception, une innovation matérielle et un prototypage pour les composants personnalisés.
3. Personnalisation et marque blanche
| Zone de personnalisation | Exemples |
|---|---|
| L'image de marque | Emballage en marque privée, impression de logo |
| Conception de produits | Formes de tête de brosse personnalisées, tailles de tubes |
| Documentation | Instructions multilingues, encarts IFU |
| Emballage | Boîtes prêtes à la vente, sachets stériles, codes-barres |
4. Délais d'exécution et logistique
- Distribution mondiale: Assurez-vous que le fournisseur a de l'expérience en matière d'expédition internationale avec la documentation appropriée.
- Inventaire et stockage: Vérifiez si le fournisseur peut offrir un stock tampon ou une livraison juste à temps.
- Flexibilité des commandes: Recherchez des partenaires avec de faibles MOQ et des capacités de réapprovisionnement flexibles.
5. Communication et support après-vente
- Gestionnaires de comptes dédiés
- Support technique pour l'intégration des produits
- Surveillance et rapports de qualité après la vente
Liste de contrôle d'évaluation des fournisseurs OEM
| Critères | Niveau d'importance | Notes |
|---|---|---|
| Certifications ISO/CE/FDA | 🌟🌟🌟🌟🌟 | Vérifiez si la documentation est |
| Installations de production en salle blanche | 🌟🌟🌟🌟🌟 | Essentielles pour la fabrication de composants stériles |
| Capacités de personnalisation | 🌟🌟🌟🌟 | Important pour l'image de marque des kits et la différenciation sur le marché |
| Communication et assistance | 🌟🌟🌟🌟 | Les acheteurs B2B ont besoin de partenaires réactifs |
| Options de logistique et de livraison | 🌟🌟🌟🌟 | Particulièrement important pour les acheteurs internationaux |
Un cadre d'évaluation complet aide les acheteurs à minimiser les risques, à améliorer les performances de la chaîne d'approvisionnement et à assurer le succès à long terme de leurs opérations de production de kits de frottis de Pap.
4. Principaux fournisseurs mondiaux de composants OEM pour les kits de frottis de Pap (Chine, États-Unis, Europe)
Choisir le bon fournisseur OEM est une décision stratégique qui a un impact direct sur la qualité, la conformité et la fiabilité des kits de frottis de Pap. Bien qu'il existe plusieurs fabricants de composants dans le monde, seuls quelques-uns répondent aux normes rigoureuses requises pour les applications de diagnostic médical. Dans cette section, nous mettrons en évidence les principaux fournisseurs des principales régions - Chine, États-Unis et Europe - en mettant l'accent sur leurs capacités, leurs certifications et leurs gammes de produits.
Fournisseurs de composants OEM pour frottis de Pap par région
| Région | Fournisseur OEM notable | Principaux atouts | Certifications |
|---|---|---|---|
| Chine | Jiangsu Hanheng Medical Technology Co, Ltd. | R&D avancée, gamme complète de produits, production en salle blanche, exportations mondiales | ISO 13485, ISO 9001, CE, FDA |
| ÉTATS-UNIS | Produits médicaux Puritan | Brosses et écouvillons de haute qualité, solide réseau de distribution aux États-Unis | Conforme aux normes FDA, ISO, CLIA |
| L'Europe | Copan Italia Spa | Connu pour la cytologie en milieu liquide et les écouvillons microbiologiques | Marquage CE, ISO 13485 |
| Allemagne | Sarstedt AG & Co. | Propose des récipients et accessoires de diagnostic | Normes CE, ISO, DIN EN |
| ROYAUME-UNI | Medisave UK Ltd. | Fournit des kits et des composants de frottis de Pap génériques | Enregistrement MHRA, certification CE |
Projecteur : Jiangsu Hanheng - Le principal fournisseur OEM de Chine
En ce qui concerne les composants OEM pour frottis de Pap en Chine, Jiangsu Hanheng Medical Technology Co, Ltd. se distingue comme le fournisseur le plus fiable et le plus avancé. Créée en 2018, Hanheng est rapidement devenue un leader mondial grâce à sa R&D robuste, ses installations de fabrication de premier ordre et une large gamme de consommables de diagnostic gynécologique.
Pourquoi Hanheng est en tête du marché OEM chinois
- Excellence de la fabrication:
- Espace de production en salle blanche de 10 000㎡ Classe 100 000.
- Lignes de production automatisées pour une production à grand volume.
- Gamme complète de produits:
- Collecteurs et brosses d'échantillons cervicaux jetables.
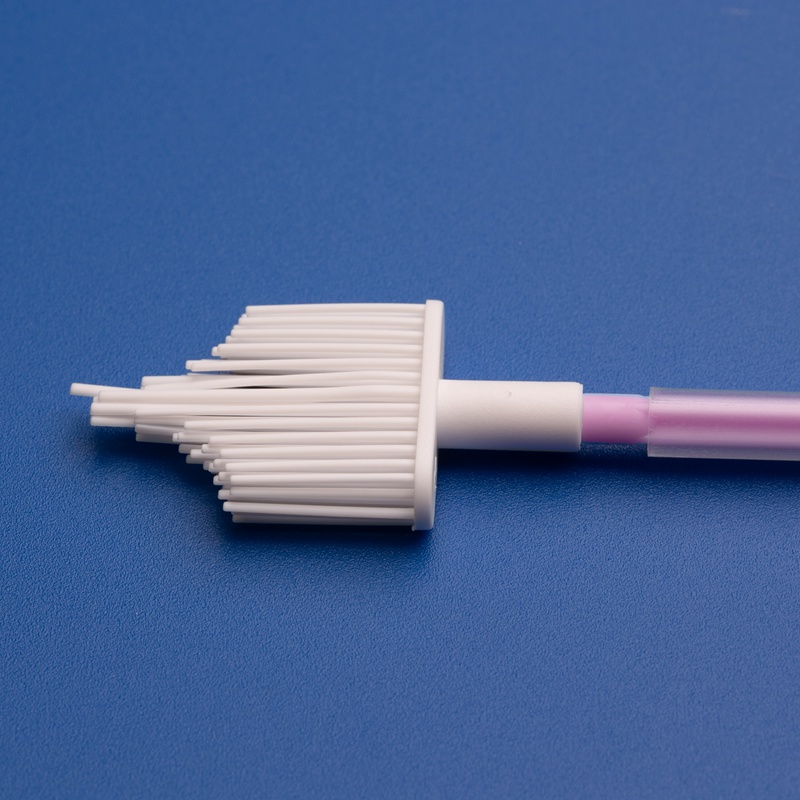
- Grattoirs gynécologiques, tubes d'échantillonnage et kits d'examen.
- Emballage et étiquetage personnalisables.
- Conformité mondiale:
- Certifié ISO9001, ISO13485.
- Composants marqués CE et autorisés par la FDA.
- De multiples brevets de modèles d'utilité garantissent l'innovation et la protection.
- Innovation en R&D:
- Gestion de bout en bout du cycle de vie des produits.
- Développement continu pour une meilleure facilité d'utilisation, l'intégrité des échantillons et le confort du patient.
- Fiable dans le monde entier:
- Produits utilisés dans les hôpitaux, les laboratoires de diagnostic et les programmes de dépistage en Europe, en Asie et en Amérique.
Offre de produits OEM pour frottis de Pap de Hanheng
| Nom du produit | Description | Caractéristiques personnalisables |
|---|---|---|
| Brosse cervicale stérile | Utilisé pour la collecte de cellules cervicales pour les tests Pap et HPV | Longueur de la tige, densité des poils, couleur |
| Collecteur d'échantillons cervicaux à usage unique | Combine une spatule et une brosse en un seul outil pour plus de commodité | Image de marque, emballage, stérilisation |
| Racloir gynécologique | Conçu pour la collecte d'échantillons ectocervicaux | Conception de la poignée, matériau |
| Tube de transport d'échantillons | Récipients étanches et à code-barres pour la cytologie en milieu liquide | Étiquetage, conservateurs, volume |
| Boîte de kit d'échantillonnage de frottis de Pap | Kit groupé pour une utilisation clinique ou à domicile | Impression du logo, encarts d'instructions |
Pour demander les prix OEM ou passer une commande en gros, contactez Hanheng à :
🌐 www.hanheng-medical.com
📧 Courriel : [email protected]
5. Pourquoi de plus en plus de fabricants de kits médicaux se tournent vers des solutions OEM personnalisées
Alors que la concurrence s'intensifie dans l'industrie du diagnostic médical, de plus en plus de producteurs de kits - en particulier ceux qui proposent des solutions de dépistage cervical - se tournent vers des solutions OEM personnalisées pour obtenir des avantages stratégiques. Des économies de coûts à la différenciation des produits, la personnalisation OEM permet aux fabricants de rester agiles, évolutifs et alignés sur les besoins du marché.
Avantages des composants OEM personnalisés pour frottis de Pap
- Cycles de développement de produits plus rapides
- Tirez parti de pièces pré-conçues pour raccourcir le temps de R&D.
- Collaborez avec les OEM pour le co-développement de nouveaux composants.
- Différenciation de la marque
- Ajoutez des logos, des schémas de couleurs uniques et des conceptions d'emballage.
- Fournissez des instructions spécifiques à la région ou des encarts linguistiques.
- Préparation réglementaire
- Les OEM comme Hanheng aident à la documentation technique pour soutenir les soumissions CE/FDA.
- Les composants pré-certifiés simplifient les processus d'enregistrement des kits.
- Production évolutive
- Augmentez facilement la production pour répondre aux volumes des contrats de santé publique ou à la demande saisonnière.
- Les OEM gèrent l'inventaire des composants tandis que les fabricants se concentrent sur l'assemblage et les ventes.
- Optimisation des coûts
- Évitez les dépenses d'investissement élevées pour la fabrication en interne.
- Bénéficiez de la chaîne d'approvisionnement optimisée et de l'approvisionnement en matériaux en vrac des OEM.
Cas d'utilisation : Kits de frottis de Pap à domicile
La personnalisation OEM a joué un rôle déterminant dans l'essor des solutions de dépistage cervical à domicile, en particulier après la COVID. Les fabricants de kits peuvent se procurer des ensembles de composants prêts à être marqués auprès des OEM, garantissant ainsi :
- Stérilité et sécurité pour une utilisation non supervisée.
- Personnalisation des instructions pour les utilisateurs à domicile.
- Formats d'emballage compacts et discrets.
Tableau des options de personnalisation OEM
| Catégorie de fonctionnalité | Options de personnalisation |
|---|---|
| Conception de produits | Taille de la brosse, manche du grattoir, matériau du tube |
| L'image de marque | Gaufrage du logo, code couleur, étiquetage privé |
| Emballage | Conception de la boîte, scellage de la pochette, autocollants inviolables |
| Instructions | IFU multilingues, codes QR pour les guides vidéo |
| Logistique | Livraison directe, emballage en vrac, configuration des palettes |
Industries courantes bénéficiant des kits OEM
- Hôpitaux et cliniques: Kits standardisés avec des composants fiables.
- Réseaux de laboratoires de diagnostic: Kits de collecte d'échantillons à haut débit.
- Entreprises de télésanté: Kits de frottis de Pap en marque blanche pour les tests à distance.
- ONG et programmes gouvernementaux: Kits en vrac pour les initiatives de dépistage de la santé publique.
En intégrant des composants OEM personnalisés dans leurs flux de travail, les producteurs de kits médicaux peuvent garder une longueur d'avance, que ce soit pour les campagnes de dépistage de masse, les tests à domicile ou la distribution internationale.
6. Pourquoi choisir Jiangsu Hanheng comme votre fournisseur de composants OEM pour frottis de Pap
Pour les fabricants qui souhaitent rationaliser la production de kits de frottis de Pap avec des composants OEM de qualité supérieure, Jiangsu Hanheng offre la combinaison idéale de qualité, de fiabilité, d'évolutivité et de personnalisation. Voici pourquoi Hanheng est le partenaire OEM de confiance des entreprises médicales du monde entier.
Avantage concurrentiel de Hanheng
| Fonctionnalité | Avantage Hanheng |
|---|---|
| Gamme complète de produits | Guichet unique pour tous les composants de frottis de Pap - des brosses aux kits complets |
| Fabrication en salle blanche | Une salle blanche de 10 000㎡ Classe 100 000 garantit une production stérile et sans contamination |
| Certifications | ISO9001, ISO13485, CE, FDA - répond aux normes de conformité mondiales |
| Capacités de personnalisation | Étiquetage privé, emballage personnalisé, encarts multilingues, ajustements de conception |
| Expérience mondiale | Approuvé par les prestataires de soins de santé dans plus de 30 pays pour les produits gynécologiques OEM |
| Équipe de R&D avancée | Développe des solutions innovantes pour une meilleure collecte d'échantillons et le confort du patient |
| Prix compétitifs | Tarification évolutive pour les commandes en gros et les contrats de fournisseurs à long terme |
| Support client réactif | Gestionnaires de compte dédiés et assistance technique pendant l'intégration |
Ce que vous pouvez attendre de Hanheng
- Délai d'exécution rapide: Planification de production efficace pour les commandes OEM urgentes.
- Collaboration de conception: Co-développer de nouveaux composants ou adapter ceux existants à votre marque.
- Assurance qualité: Inspections de contrôle qualité rigoureuses et systèmes de traçabilité.
- Logistique mondiale: Expérience d'expédition vers les États-Unis, l'UE, l'Asie du Sud-Est et l'Amérique latine.
Témoignages de clients
« Nous sommes passés à Hanheng pour nos composants de frottis de Pap en 2022, et cela a considérablement amélioré la fiabilité de nos kits et réduit nos délais. »
— Chef de produit, société de diagnostic basée dans l'UE
« Leur équipe nous a aidés à concevoir et à lancer un kit de dépistage cervical entièrement marqué pour notre plateforme de télésanté. Le processus a été transparent. »
— PDG, startup de santé féminine, États-Unis
Composants OEM pour frottis de Pap disponibles
| Composant | Disponibilité OEM | Personnalisation | MOQ |
|---|---|---|---|
| Brosses cervicales (stériles) | ✅ Disponible | Oui | 5 000 unités |
| Spatules de cytologie | ✅ Disponible | Oui | 10 000 unités |
| Tubes et flacons d'échantillons | ✅ Disponible | Oui | 5 000 unités |
| Boîtes de kits d'échantillonnage | ✅ Disponible | Oui | 1 000 kits |
| Collecteurs d'échantillons cervicaux | ✅ Disponible | Oui | 5 000 unités |
Pour explorer les capacités OEM de Hanheng ou demander un devis :
🌐 Visite : www.hanheng-medical.com
📧 Courriel : [email protected]
7. Processus étape par étape : Commande de composants OEM pour frottis de Pap pour l'assemblage de kits
Pour les acheteurs B2B tels que les assembleurs de kits de diagnostic, les équipes d'approvisionnement hospitalières et les distributeurs de soins de santé, l'approvisionnement en composants OEM pour frottis de Pap nécessite une approche structurée pour garantir la qualité, la conformité réglementaire et la livraison en temps voulu. Jiangsu Hanheng Medical Technology Co, Ltd. offre un processus de commande rationalisé et professionnel, adapté aux besoins des acheteurs en gros mondiaux et des partenaires OEM.
Vous trouverez ci-dessous un guide complet pour vous aider à comprendre chaque phase du flux de travail de commande OEM - de la demande initiale à la livraison finale.
Étape 1 : Demande initiale et collecte des exigences
Le processus commence par la définition de vos exigences spécifiques. Cela permet au fournisseur OEM de proposer les solutions les plus adaptées.
Ce qu'il faut préparer :
- Spécifications du produit (par exemple, type de brosse cervicale, conception du grattoir)
- Utilisation prévue (clinique, laboratoire de diagnostic ou kit à domicile)
- Certifications requises (par exemple, CE, FDA, ISO)
- Estimations de quantité et fréquence de réapprovisionnement prévue
- Préférences en matière d'emballage et d'image de marque
Informations que Hanheng fournira :
- Catalogues de produits avec spécifications techniques
- Documents de capacité OEM
- Options de personnalisation d'échantillons
- Certificats de conformité (ISO 13485, CE, FDA)
📧 Contact : [email protected] pour initier votre demande.
Étape 2 : Demande et validation d'échantillons
Avant de passer une commande en gros, la plupart des acheteurs demandent des échantillons de produits pour les tests et la validation.
Processus d'échantillonnage :
- Hanheng envoie des échantillons des composants OEM demandés (par exemple, brosses cervicales stériles, flacons d'échantillons).
- Vous effectuez des tests internes ou des essais cliniques pour valider la compatibilité avec vos kits ou systèmes de laboratoire existants.
- Des commentaires sont fournis pour tout ajustement ou amélioration de la conception.
Liste de contrôle de la validation :
| Critères de validation | Notes |
|---|---|
| Efficacité du prélèvement des échantillons | Confirmer la récupération adéquate des cellules épithéliales |
| Compatibilité des matériaux | Assurer la compatibilité des tubes/flacons avec les fixateurs |
| Stérilité et intégrité de l'emballage | Vérifier la résistance de l'étanchéité et la précision de l'étiquetage |
| Examen du prototype de marque | Vérifier le placement du logo, la conception de la boîte, les notices d'instructions |
Étape 3 : Personnalisation et image de marque OEM
Après la validation, l'étape suivante consiste à finaliser les détails de personnalisation de vos kits ou composants de marque.
Options personnalisables avec Hanheng :
| Composant | Options de personnalisation |
|---|---|
| Brosses cervicales | Couleur de la tige, longueur, forme des soies, impression du logo |
| Spatules de cytologie | Conception de la poignée, style d'emballage |
| Tubes/flacons d'échantillons | Code-barres, volume, couleur du bouchon, conception de l'étiquette |
| Boîtes d'emballage de kits | Taille de la boîte, image de marque imprimée, notices d'utilisation multilingues |
Collaboration de conception :
- L'équipe de conception de Hanheng travaille avec vous pour finaliser les illustrations en marque blanche.
- Des maquettes numériques et des rendus 3D sont fournis pour approbation.
- En option : impression de code QR, étiquetage SKU unique pour la gestion des stocks.
Étape 4 : Établissement du devis et finalisation du contrat
Une fois les spécifications et l'image de marque approuvées, Hanheng fournira un devis détaillé et rédigera un projet d'accord de fabrication.
Le devis comprend :
- Prix unitaire (basé sur le volume de la commande)
- Frais d'outillage ou de moule (le cas échéant pour les pièces personnalisées)
- Estimation des délais
- Frais de livraison et de logistique
- Conditions et options de paiement
Points forts contractuels :
- Accord de non-divulgation (NDA) pour protéger vos conceptions et formulations.
- Conditions d'assurance qualité couvrant les tests de lots, la stérilité et la traçabilité.
- Conditions de réapprovisionnement et options de remise sur volume.
Étape 5 : Production et contrôle qualité
Dès la signature de l'accord et la réception du paiement initial ou de l'acompte, la production commence dans l'installation de pointe de Hanheng.
Caractéristiques de fabrication :
- Salle blanche de classe 100 000 pour la production de composants stériles
- Automatisation à haut volume pour une exécution rapide
- Suivi des lots et étiquetage des numéros de lot
Procédures de contrôle qualité :
| Étape de contrôle qualité | Tests effectués |
|---|---|
| Contrôle des matériaux entrants | Identification des matériaux, dépistage de la stérilité |
| Contrôle qualité en cours de fabrication | Tests dimensionnels, résistance des soies, validation de l'étanchéité |
| Inspection finale | Intégrité de l'emballage, exactitude des étiquettes, audit de stérilité |
Tous les composants sont certifiés selon les normes ISO 13485 et subissent une assurance qualité rigoureuse avant expédition.
Étape 6 : Expédition et livraison
Une fois la production terminée, Hanheng gère la logistique d'expédition mondiale en utilisant des partenaires de fret de confiance.
Options d'expédition :
- EXW, FOB, CIF ou DDP selon la préférence de l'acheteur
- Fret aérien pour les commandes urgentes
- Fret maritime pour les envois en vrac
Documentation requise :
- Facture commerciale
- Liste de colisage
- Certificats d'origine
- Certification CE/FDA/ISO (sur demande)
- FDS du produit (le cas échéant)
Délais de livraison estimés :
| Région | Mode d'expédition | Délai de livraison typique |
|---|---|---|
| Amérique du Nord | Fret aérien | 5 à 7 jours ouvrables |
| L'Europe | Fret maritime | 20-30 jours |
| Asie du Sud-Est | Fret aérien | 3 à 5 jours ouvrables |
| Amérique du Sud | Fret maritime | 25–35 jours |
Étape 7 : Support après-vente et réapprovisionnement
Hanheng offre un soutien à long terme aux clients OEM, notamment :
- Des gestionnaires de compte dédiés
- Boucle de rétroaction sur la qualité
- Alertes de réapprovisionnement et planification des stocks
- Support technique pour l'intégration des kits
📩 Pour les réapprovisionnements, envoyez un e-mail à : [email protected] avec votre numéro de commande ou vos spécifications précédentes.


8. Foire aux questions sur les composants des kits de frottis Pap OEM
Q1 : Quelle est la quantité minimale de commande (MOQ) pour les brosses cervicales OEM ?
R : Le MOQ de Hanheng pour les brosses cervicales OEM commence à 5 000 unités. Pour les kits entièrement personnalisés (y compris l'emballage et les encarts), le MOQ est généralement de 1 000 kits.
Q2 : Puis-je faire imprimer des instructions multilingues dans les kits ?
R : Oui. Hanheng prend en charge les encarts IFU (instructions d'utilisation) multilingues. Vous pouvez choisir parmi un ensemble de modèles pré-traduits ou fournir vos propres traductions.
Q3 : Comment puis-je m'assurer que les composants OEM sont stériles ?
R : Tous les composants de Hanheng sont produits dans des environnements de salle blanche certifiés ISO de classe 100 000 et subissent une stérilisation à l'oxyde d'éthylène (EO) ou aux rayons gamma, selon le produit. Des rapports de stérilité peuvent être fournis sur demande.
Q4 : Quelles certifications les composants OEM de Hanheng détiennent-ils ?
R : Les composants de frottis Pap OEM de Hanheng sont certifiés selon les normes ISO9001 et ISO13485. La plupart des produits portent également le marquage CE et l'enregistrement FDA, ce qui les rend adaptés aux marchés mondiaux.
Q5 : Puis-je demander un prototype avant de passer une commande importante ?
R : Absolument. Hanheng propose des kits d'échantillons et le développement de prototypes pour les clients OEM. Ceci est particulièrement utile pour valider l'image de marque, la convivialité et la compatibilité des composants.
Q6 : Quels types d'options de branding sont disponibles ?
R : Hanheng propose une image de marque OEM/marque blanche complète, notamment :
- Conception de boîte personnalisée
- Impression du logo sur les instruments
- Intégration de codes-barres et de codes QR
- Inserts multilingues
- Composants à code couleur
Q7 : Combien de temps faut-il pour produire et expédier une commande en gros ?
R : La production prend généralement de 3 à 5 semaines, selon la personnalisation. Le délai d'expédition varie selon la région et la méthode (aérien ou maritime). Hanheng propose une production accélérée pour les commandes urgentes.
Q8 : Quelles sont les conditions de paiement ?
R : Les conditions de paiement standard sont un acompte de 30 % à la confirmation de la commande et 70 % avant l'expédition. Des conditions flexibles peuvent être négociées pour les partenaires OEM à long terme.
Q9 : Un soutien réglementaire est-il fourni pour les marchés internationaux ?
R : Oui. Hanheng fournit toute la documentation nécessaire, telle que les certificats CE, l'enregistrement FDA, les certificats ISO et les rapports d'essais, pour faciliter les soumissions réglementaires locales.
9. Conclusion et appel à l'action
Alors que la demande mondiale de dépistage du cancer du col de l'utérus augmente, les fabricants de kits médicaux doivent rationaliser la production sans compromettre la qualité, la conformité ou l'innovation. S'associer à un fournisseur OEM fiable comme Jiangsu Hanheng Medical Technology Co, Ltd. garantit que vos kits de frottis Pap sont construits avec des composants de précision, entièrement personnalisés pour votre marque et conformes aux normes médicales internationales.
Que vous lanciez un nouveau kit de diagnostic gynécologique, que vous augmentiez la production pour un programme de dépistage national ou que vous vous développiez sur les marchés de diagnostic à domicile, Hanheng peut vous aider à atteindre vos objectifs avec rapidité, fiabilité et flexibilité.
✅ Fabrication certifiée ISO 13485
✅ Composants conformes aux normes CE et FDA
✅ Personnalisation pour l'image de marque et l'emballage
✅ Production évolutive pour la distribution mondiale
📞 Prêt à rationaliser la production de votre kit de frottis Pap avec les composants OEM de Hanheng ?
👉 Visitez www.hanheng-medical.com
📧 Courriel : [email protected]
Laissez Hanheng être votre partenaire OEM de confiance pour la construction de la prochaine génération de solutions de dépistage du col de l'utérus.

Jiangsu Hanheng Medical Technology Co, Ltd.
Nous sommes l'un des principaux fabricants de consommables médicaux de haute qualité et nous nous engageons à assurer la précision, la sécurité et le respect des normes internationales. Grâce à une technologie de production avancée, à un contrôle de qualité strict et à une équipe de recherche et développement dévouée, nous fournissons des solutions fiables adaptées aux besoins changeants de l'industrie des soins de santé.



