Usine de brosses cytologiques médicales jetables en Europe de l'Est

Partager
1. Introduction : La demande croissante pour les brosses cytologiques médicales jetables
Au cours de la dernière décennie, les diagnostics médicaux et la santé préventive sont devenus des domaines de focalisation clés pour les systèmes de santé nationaux et les cliniques privées. Les dépistages cytologiques, en particulier ceux pour cancer du col de l'utérus, ont gagné en prominence en tant que mesure préventive essentielle. Cela a stimulé la demande pour des brosses cytologiques médicales jetables de haute qualité qui permettent une collecte précise, stérile et efficace d'échantillons cervicaux.
Qu'est-ce qu'une brosse cytologique médicale jetable ?
Une brosse cytologique jetable est un dispositif médical stérile à usage unique utilisé pour collecter des échantillons cellulaires du col de l'utérus pour les frottis Pap et les tests VPH. Ces brosses sont conçues pour assurer une efficacité maximale de collecte d'échantillons tout en minimisant l'inconfort pour le patient.
Applications en milieu médical
- Examens gynécologiques: Pour les tests Pap de routine et les dépistages VPH.
- Dépistages du cancer: Détection et surveillance du cancer du col de l'utérus.
- Laboratoires cliniques: Utilisées dans les laboratoires de cytopathologie pour l'analyse d'échantillons.
- Hôpitaux et cliniques de santé des femmes: Essentielles pour les départements d'obstétrique-gynécologie.
Pourquoi la demande augmente
L'augmentation des campagnes de sensibilisation au cancer du col de l'utérus et l'expansion des programmes nationaux de dépistage — en particulier dans les pays d'Europe de l'Est — ont contribué de manière significative à la hausse de la demande. De plus, le passage d'outils réutilisables à des brosses cytologiques jetables en raison des protocoles de contrôle des infections a accéléré cette tendance.
Moteurs du marché
| Moteur du marché | Description |
|---|---|
| Expansion des programmes de dépistage | Les programmes nationaux de dépistage du cancer du col de l'utérus augmentent en fréquence. |
| Protocoles de contrôle des infections | Les hôpitaux passent à des outils jetables pour réduire la contamination croisée. |
| Croissance des cliniques privées | Augmentation des pratiques privées d'obstétrique-gynécologie et des cliniques diagnostiques. |
| Réglementations des dispositifs médicaux de l'UE | Des exigences plus strictes favorisent les jetables de haute qualité et certifiés. |
2. Tendances du marché des consommables cytologiques en Europe de l'Est
L'Europe de l'Est connaît une transformation de son paysage de la santé. Des pays comme la Pologne, la Roumanie, la Bulgarie et la Hongrie investissent massivement dans les infrastructures de santé publique, y compris les programmes de dépistage du cancer du col de l'utérus et les services diagnostiques. Cela a conduit à un marché en croissance pour les consommables cytologiques, incluant les brosses jetables.
Statistiques clés du marché
- Financement de l'UE: De nombreuses nations d'Europe de l'Est reçoivent des fonds de l'UE pour améliorer les normes de santé, y compris les tests cytologiques.
- Taux de dépistage accrus: Selon les données de l'OMS, les taux de dépistage du cancer du col de l'utérus ont augmenté de plus de 30 % dans certains pays de 2018 à 2023.
- Importations en hausse: Les volumes d'importation de consommables gynécologiques ont crû à un TCAC de 6,5 % en Europe de l'Est.
Aperçu par pays
| Pays | Couverture de dépistage (%) | Maturité du marché | Programmes gouvernementaux |
|---|---|---|---|
| Pologne | 63% | Élevée | Programme national de lutte contre le cancer du col de l'utérus |
| Roumanie | 52% | Moyen | Initiatives de dépistage du VPH |
| Bulgarie | 47% | Bas-moyen | Croissance des diagnostics public-privé |
| Hongrie | 68% | Élevée | Tests préventifs subventionnés |
| République tchèque | 71% | Élevée | Dépistages du cancer soutenus par le gouvernement |
Tendances à surveiller
- Localisation de la production: L'Europe de l'Est encourage l'établissement d'usines locales de dispositifs médicaux pour réduire la dépendance aux importations.
- Conformité au RDM de l'UE: Les usines intensifient leurs efforts pour se conformer aux règlements européens sur les dispositifs médicaux (RDM).
- Fabrication OEM et marque de distributeur: Un intérêt croissant de l'Europe occidentale et de l'Amérique du Nord pour s'approvisionner en brosses cytologiques auprès d'usines rentables en Europe de l'Est.
Zones d'opportunités B2B
- Services d'approvisionnement hospitaliers: Recherche de contrats à long terme avec des fournisseurs fiables.
- Distributeurs de dispositifs médicaux: À la recherche de brosses cytologiques conformes et à prix compétitif.
- Plateformes B2B de commerce électronique: Fourniture de cliniques privées et de laboratoires.
- Soumissionnaires aux appels d'offres gouvernementaux: Besoin de dispositifs cytologiques certifiés RDM pour les programmes de santé publique.

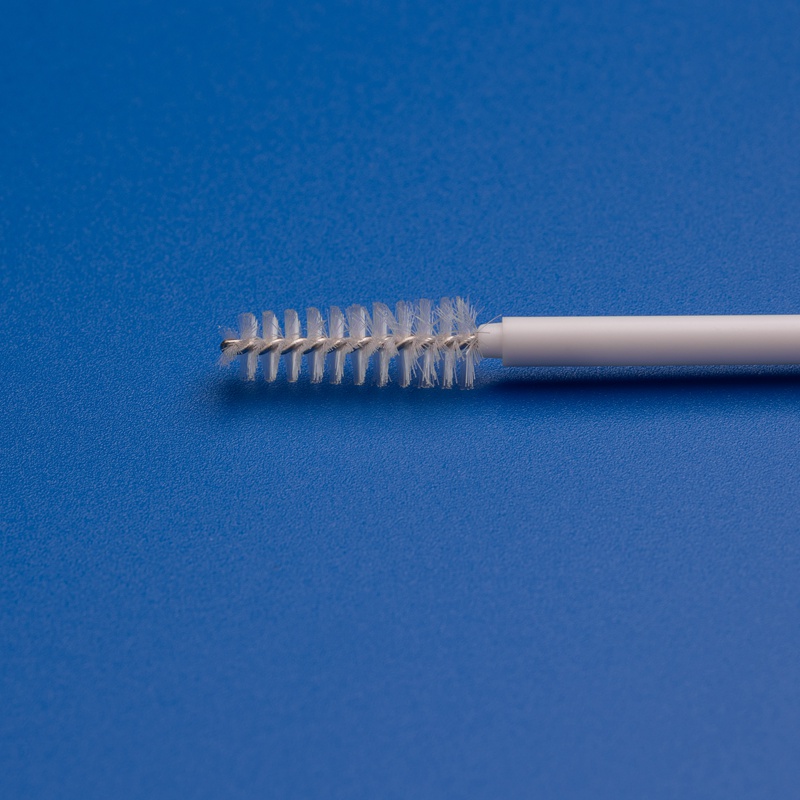

3. Facteurs clés à considérer lors du choix d'un fabricant de brosses cytologiques
Avec un marché des consommables médicaux de plus en plus concurrentiel, sélectionner le bon fabricant pour les brosses cytologiques jetables est crucial pour les responsables des achats, les distributeurs et les acheteurs en santé. Voici les facteurs essentiels à examiner.
1. Conformité réglementaire
Assurez-vous que le fabricant respecte les normes internationales, notamment :
- ISO 13485: Système de gestion de la qualité pour les dispositifs médicaux
- Certification CE: Requis pour la distribution au sein de l'Espace économique européen (EEE)
- Enregistrement auprès de la FDA (si exportation vers les États-Unis)
2. Qualité et conception du produit
Les brosses cytologiques de haute qualité doivent offrir :
- Prélèvement de précision: Conception optimisée des poils pour une collecte cellulaire efficace
- Assurance de stérilisation: Stérilisées à l'oxyde d'éthylène et emballées individuellement
- Confort du patient: Conception de manche lisse et ergonomique
- Compatibilité: Adaptées à une utilisation avec des équipements de laboratoire standard
Tableau des spécifications du produit
| Fonctionnalité | Spécification |
|---|---|
| Matériau de la brosse | Poils en nylon ou polyester |
| Matériau du manche | ABS ou polypropylène de qualité médicale |
| Méthode de stérilisation | Oxyde d'éthylène (EO) |
| Durée de conservation | 3-5 ans (emballage scellé) |
| Emballage | Conditionnés individuellement, 100 unités par carton |
| Certification | CE, ISO13485, FDA (varie selon le fabricant) |
3. Capacités de fabrication
Évaluer la capacité de production et les compétences technologiques de l'usine :
- Installations en salle blanche: Au moins la classe ISO 7 (100 000) est préférable
- Niveau d'automatisation: Un haut niveau d'automatisation garantit la cohérence des produits
- Capacités de R&D: Capacité d'innovation et de personnalisation pour les clients OEM
- Évolutivité: Peut répondre à des commandes à faible MOQ et à grand volume
4. Logistique et délais de livraison
Les fabricants fiables offrent :
- MOQ flexibles: Adapté aux petits distributeurs comme aux grandes institutions
- Délai d'exécution rapide: Délai de livraison standard inférieur à 30 jours
- Expérience de l'expédition mondiale: Expertise en documentation, dédouanement et fret
5. Services de support B2B
Recherchez des fournisseurs proposant :
- Services OEM/ODM pour marque privée
- Support commercial multilingue
- Gestionnaires de comptes dédiés
- Assistance pour la documentation réglementaire
4. Top 5 des usines de brosses cytologiques médicales jetables en Europe de l'Est
L'Europe de l'Est abrite plusieurs fabricants réputés de dispositifs médicaux spécialisés dans les consommables gynécologiques, y compris les brosses cytologiques jetables. Ces usines offrent des prix compétitifs, une expédition rapide à travers l'Europe et une conformité aux réglementations européennes sur les dispositifs médicaux. Voici une liste sélectionnée des 5 principaux fabricants de brosses cytologiques jetables en Europe de l'Est, idéale pour les acheteurs B2B, les grossistes et les distributeurs médicaux.
1. Polmed Sp. z o.o. (Pologne)
Aperçu: L'un des plus grands fabricants de dispositifs médicaux en Pologne, Polmed propose une gamme complète de produits gynécologiques tels que les brosses cervicales, les kits pour frottis Pap et les kits d'examen.
- Certifications: ISO 13485, CE
- Spécialisations:
- Brosses cytologiques pour test Pap
- Brosses endocervicales et combinées
- Options de marque privée
- MOQ: 10 000 pièces
- Délai d'exécution: 2 à 3 semaines
- Focus B2B: Hôpitaux, laboratoires de diagnostic, distributeurs régionaux
2. Rommedica Medical SRL (Roumanie)
Aperçu: Rommedica est une usine roumaine de consommables médicaux qui met l'accent sur les outils gynécologiques stériles. Elle approvisionne les Balkans et l'Europe centrale.
- Certifications: CE, ISO 9001
- Gamme de produits:
- Brosses cytologiques jetables stériles
- Kits d'échantillonnage pour tests HPV et Pap
- MOQ: 5 000 pièces
- Délai d'exécution: 3-4 semaines
- Services B2B:
- Une image de marque personnalisée
- La fabrication OEM
- Support à la formation des distributeurs
3. MediEstetika Ltd. (Bulgarie)
Aperçu: Située à Sofia, MediEstetika est reconnue pour ses consommables gynécologiques de haute qualité, avec un accent sur l'abordabilité et la conformité à l'UE.
- Certifications: ISO 13485, Marquage CE
- Principaux produits:
- Cytobrosse cervicale avec poignées ergonomiques
- Systèmes à double brosse pour échantillonnage endo/exocervical
- MOQ: 3 000 pièces
- Délai d'exécution: 2 à 3 semaines
- Offres B2B: Échelles de prix pour distributeurs, livraison rapide dans toute l'UE
4. Medifem Kft. (Hongrie)
Aperçu: Medifem agit à la fois comme fabricant et centre de recherche pour les diagnostics de la santé des femmes. Ses brosses cytologiques sont largement utilisées dans les hôpitaux hongrois et exportées vers l'Europe occidentale.
- Certifications: CE, ISO 13485
- Avantages des produits:
- Poils à pointe souple pour le confort du patient
- Longueurs d'axe personnalisables
- MOQ: 2 000 pièces
- Délai d'exécution: 2 semaines
- Canaux de distribution:
- Contrats directs avec les hôpitaux
- Appels d'offres nationaux
5. Cytomed d.o.o. (Slovénie)
Aperçu: Cytomed est une entreprise de technologie médicale qui collabore avec des universités régionales pour la R&D et produit des brosses cytologiques de haute précision.
- Certifications: ISO 13485, CE
- Caractéristiques uniques:
- Options biodégradables disponibles
- Designs améliorant l'efficacité d'échantillonnage
- MOQ: 5 000 pièces
- Délai d'exécution: 3 semaines
- Valeur B2B:
- Hôpitaux axés sur la durabilité
- Cliniques en quête d'innovation
Tableau comparatif récapitulatif
| Fabricant | Pays | Certifications | MOQ | Délai d'exécution | Services OEM |
|---|---|---|---|---|---|
| Polmed | Pologne | ISO 13485, CE | 10,000 | 2 à 3 semaines | Oui |
| Rommedica | Roumanie | CE, ISO 9001 | 5,000 | 3 à 4 semaines | Oui |
| MediEstetika | Bulgarie | ISO 13485, CE | 3,000 | 2 à 3 semaines | Oui |
| Medifem | Hongrie | CE, ISO 13485 | 2,000 | 2 semaines | Oui |
| Cytomed | Slovénie | ISO 13485, CE | 5,000 | 3 semaines | Oui (respectueux de l'environnement) |
5. Pourquoi l'Europe de l'Est devient un hub stratégique pour les consommables médicaux
L'Europe de l'Est émerge rapidement comme un hub stratégique pour la production et l'exportation de consommables médicaux tels que les brosses cytologiques jetables. Ce changement est motivé par une combinaison de conditions économiques favorables, d'une main-d'œuvre qualifiée et de la proximité des principaux marchés de la santé au sein de l'Union européenne.
Avantages stratégiques clés
1. Fabrication économique
- Coûts de main-d'œuvre: Les pays d'Europe de l'Est offrent des coûts de main-d'œuvre significativement inférieurs à ceux de l'Europe occidentale.
- Frais d'exploitation: Des coûts immobiliers et d'utilités plus bas réduisent les dépenses de production globales.
- Avantage tarifaire: Permet aux fabricants d'offrir des prix B2B compétitifs pour les acheteurs en gros.
2. Accès au marché de l'UE
- Zones de libre-échange: Les fabricants bénéficient d'un commerce sans droits de douane au sein de l'UE.
- Réglementations harmonisées: Les produits fabriqués selon les normes MDR de l'UE peuvent être vendus dans les 27 États membres de l'UE.
- Expédition rapide: La localisation en Europe centrale assure une livraison rapide aux hôpitaux, cliniques et laboratoires.
3. Main-d'œuvre qualifiée
- Ingénierie des dispositifs médicaux: Les universités de la région forment des ingénieurs et techniciens hautement qualifiés.
- Équipes de vente multilingues: Facilite la communication B2B internationale et le support technique.
4. Soutien gouvernemental
- Subventions et subventions: De nombreux gouvernements financent l'encouragement des exportations de dispositifs médicaux.
- Incitations à l'exportation: Encouragement aux partenariats OEM et aux coentreprises avec des entreprises étrangères.
5. Écosystème de R&D en croissance
- Collaboration université-industrie: De nombreuses usines s'associent avec des facultés de médecine pour l'innovation.
- Accent sur la durabilité: Un nombre croissant de fabricants développent des brosses cytologiques écologiques et biodégradables.
6. Pourquoi choisir Jiangsu Hanheng comme fournisseur de brosses cytologiques basé en Chine
Bien que l'Europe de l'Est offre une proximité aux acheteurs de l'UE, la Chine reste une puissance mondiale dans la fabrication de consommables médicaux. Pour les acheteurs en quête d'approvisionnement fiable à grand volume avec une innovation de pointe, Jiangsu Hanheng Medical Technology Co, Ltd. est le premier choix en Chine.
À propos de Jiangsu Hanheng
Fondée en 2018, Jiangsu Hanheng est un fabricant leader de consommables pour tests médicaux. L'entreprise exploite une salle blanche de classe 100 000 de 10 000 m² et exporte mondialement avec toutes les certifications réglementaires.
Pourquoi les acheteurs mondiaux font confiance à Hanheng
| Fonctionnalité | Avantage de Jiangsu Hanheng |
|---|---|
| Conformité réglementaire | CE, ISO 13485, FDA, plusieurs brevets de modèle d'utilité |
| Excellence de la fabrication | Installations avancées, lignes de production automatisées |
| Innovation en R&D | Équipe dédiée à l'optimisation et au développement des produits |
| Gamme de produits | Portefeuille complet d'échantillonnage gynécologique incluant les brosses cytologiques |
| Personnalisation et services OEM | Capacités complètes de marque privée pour distributeurs mondiaux |
| Soutien logistique mondial | Expédition internationale rapide, fiable et économique |
Points forts des produits
- Brosses cytologiques jetables stériles: Conçues pour un prélèvement précis d'échantillons cervicaux avec des poils à pointe souple et des poignées ergonomiques.
- Collecteurs d'échantillons cervicaux: Compatibles avec divers systèmes de diagnostic.
- Trousses d'examen gynécologique: Kits complets pour cliniques et hôpitaux.
Services B2B
- Fabrication OEM/ODM avec emballage personnalisé
- Échelles de prix en gros pour distributeurs
- Documentation technique et support réglementaire
- Équipe de vente dédiée, fluent en anglais et expérimentée en B2B mondial
Idéal pour
- Distributeurs médicaux internationaux
- Fournisseurs de programmes de dépistage du cancer du col de l'utérus
- Revendeurs de produits de laboratoire
- Responsables des achats hospitaliers
📩 Pour demander des brosses cytologiques en gros ou des échantillons, contactez Jiangsu Hanheng à [email protected] ou visitez le site web officiel à www.hanheng-medical.com
7. Comment approvisionner des brosses cytologiques en gros depuis l'Europe de l'Est et la Chine
Que vous soyez distributeur, responsable des achats hospitaliers ou marque privée, l'approvisionnement en brosses cytologiques jetables à prix de gros nécessite une approche stratégique. Avec l'Europe de l'Est et la Chine comme deux des régions les plus proéminentes pour la fabrication de consommables médicaux, comprendre le processus d'approvisionnement peut rationaliser les achats et assurer qualité, conformité et fiabilité.
Guide étape par étape pour l'approvisionnement en brosses cytologiques en gros
Étape 1 : Définir vos besoins
Avant de contacter les fabricants, définissez clairement :
- Spécifications du produit: Type de poils, taille de la tête de brosse, longueur de l'axe, méthode de stérilisation
- Certifications requises: CE, FDA, ISO 13485 selon votre marché
- Préférences d'emballage: Emballages stériles individuels, options en vrac, marque privée
- MOQ et volume: Définissez votre quantité de commande initiale et vos volumes mensuels/trimestriels
- Délais de livraison: Planifiez les délais de production, surtout pour les appels d'offres publics ou les essais cliniques
Étape 2 : Établir une liste restreinte de fabricants qualifiés
Utilisez des plateformes B2B et des ressources pour identifier des fournisseurs conformes et expérimentés :
| Plateforme/Ressource | Description |
|---|---|
| Foire Medica | Exposition mondiale de dispositifs médicaux pour rencontrer des fournisseurs |
| Alibaba / Made-in-China | Plateformes B2B avec fabricants vérifiés |
| Annuaires d'entreprises de l'UE | Listes de fabricants par certification et produit |
| Site web officiel de Hanheng | Contact direct pour échantillons et devis : www.hanheng-medical.com |
| Réseaux de distributeurs | Demandez des recommandations à vos contacts B2B existants |
Étape 3 : Demander des échantillons et des documents
Contactez les fabricants présélectionnés pour demander :
- Des échantillons de produits
- Certificats de qualité et de stérilisation
- Documentation CE, ISO, FDA
- Rapports d'audit d'usine (si disponibles)
- Brochures de capacités OEM/ODM
Étape 4 : Évaluer et comparer
Créez une matrice de comparaison pour évaluer les fournisseurs en fonction de :
| Critères | Poids (%) | Notes |
|---|---|---|
| Qualité du produit | 30% | Performance des échantillons, design, stérilité |
| Certifications et conformité | 20% | CE/FDA pour l'entrée sur le marché |
| Coût par unité | 15% | Considérez le coût total livré |
| Délai d'exécution | 10% | Jours de la commande à la livraison |
| Capacités OEM/Marque privée | 10% | Options de personnalisation |
| Communication et service | 10% | Réactivité, professionnalisme |
| Assistance logistique | 5% | Incoterms, expérience en expédition |
Étape 5 : Négocier les termes et passer une commande d'essai
Une fois un fournisseur sélectionné, négociez :
- Conditions de paiement (généralement 30 % d'acompte, 70 % avant expédition)
- Incoterms de livraison (EXW, FOB, DDP, etc.)
- Options de marque et d'emballage personnalisées
- Processus d'assurance qualité
- Assistance après-vente
Commencez par une commande d'essai (par ex., 5 000–10 000 unités) pour vérifier la cohérence avant de passer à l'échelle.
Approvisionnement en Europe de l'Est vs. Chine : Une comparaison
| Facteur | Europe de l'Est | Chine (par exemple, Hanheng) |
|---|---|---|
| Proximité à l'UE | Proximité étroite, fret plus bas pour les acheteurs de l'UE | Temps d'expédition plus longs pour l'UE, plus rapides pour l'Asie/Afrique |
| Conformité réglementaire | Usines conformes au MDR de l'UE | Offre CE, FDA, ISO 13485, acceptés mondialement |
| Coûts de fabrication | Modérée à élevée | Coûts de fabrication et de main-d'œuvre plus bas |
| Innovation et R&D | Innovation modérée | Forte R&D, surtout Hanheng dans les solutions cytologiques |
| Services OEM/Marque privée | Disponible, mais plus limité | Complet, évolutif et personnalisable |
| Évolutivité | Capacité de production limitée dans certaines usines | Production à grand volume avec des lignes automatisées |
| Quantités minimum de commande | Inférieur dans certains cas | Modéré, mais flexible (Hanheng propose des MOQ bas) |
| Délai d'exécution | 2–4 semaines | 3 à 5 semaines (avion) ou 30 à 40 jours (transport maritime) |
Meilleures pratiques pour les acheteurs en gros
- Utilisez judicieusement les Incoterms: Négociez les termes EXW ou FOB en fonction de votre réseau logistique.
- Planifiez les cycles d'inventaire: Passez des commandes d'achat échelonnées pour maintenir un approvisionnement régulier.
- Auditez les fournisseurs: Si possible, effectuez des audits par des tiers ou des inspections vidéo.
- Tirez parti des certifications: Utilisez la documentation CE/FDA pour faciliter les douanes et la conformité.
- Construisez des relations: Des relations solides avec les fournisseurs mènent à de meilleurs termes de crédit et à une production prioritaire.

8. Questions fréquemment posées sur l'achat de brosses cytologiques en vrac
Voici les réponses aux questions courantes posées par les acheteurs en gros, les agents d'approvisionnement et les distributeurs médicaux à la recherche de brosses cytologiques jetables.
Q1 : Quelles certifications dois-je rechercher chez un fournisseur de brosses cytologiques ?
R: Les certifications les plus importantes incluent :
- ISO 13485: Système de gestion de la qualité pour les dispositifs médicaux
- Marque CE: Requis pour la distribution au sein de l'Union européenne
- Enregistrement auprès de la FDA: Requis pour le marché américain
- Rapports de validation de stérilisation: Validation de stérilisation à l'oxyde d'éthylène ou gamma
Hanheng, par exemple, détient les certifications CE, FDA, ISO 13485 et plusieurs brevets de modèle d'utilité.
Q2 : Quel est le MOQ typique pour les commandes en gros de brosses cytologiques ?
R: Les quantités minimum de commande varient selon la région et le fabricant :
- Europe de l'Est: 2 000–5 000 pièces
- Chine (Hanheng): MOQ flexibles à partir de 5 000 unités, évolutifs jusqu'à 100 000+
Q3 : Puis-je marquer les brosses cytologiques avec le logo de ma clinique ou de mon distributeur ?
R: Oui. La plupart des fabricants, y compris Jiangsu Hanheng, proposent :
- OEM et ODM complets
- Emballage et étiquetage personnalisés
- Instructions et IFU multilingues
- Assistance à la conception de la marque
Q4 : Combien de temps faut-il pour recevoir les commandes ?
R: Les délais de livraison dépendent du volume de la commande et du mode d'expédition :
| Région | Temps de production | Temps d'expédition (Avion) | Temps d'expédition (Mer) |
|---|---|---|---|
| Europe de l'Est | 2–4 semaines | 2–5 jours (au sein de l'UE) | N/D |
| Chine (Hanheng) | 3–5 semaines | 5-10 jours | 30–40 jours |
Q5 : Les brosses cytologiques sont-elles à usage unique ou réutilisables ?
R: Toutes les brosses cytologiques discutées ici sont des dispositifs stériles à usage unique conçus pour un usage unique afin de prévenir la contamination croisée et d'assurer la sécurité des patients.
Q6 : Quelles options d'emballage sont disponibles ?
R: Les formats d'emballage courants incluent :
- Emballage stérile individuel (blister ou emballage flow wrap)
- Emballage en vrac (pour les institutions disposant de capacités de champ stérile)
- Kits OEM (incluant brosse, spatule et conteneur d'échantillon)
Q7 : Puis-je obtenir des échantillons avant de passer une commande en gros ?
R: Oui. La plupart des fabricants, y compris Hanheng, proposent des échantillons gratuits pour évaluation. Contactez [email protected] pour demander le vôtre.
9. Conclusion : S'associer à des fabricants de confiance pour un succès à long terme
Alors que la demande mondiale pour le dépistage du cancer du col de l'utérus et les diagnostics gynécologiques continue de croître, le besoin de brosses cytologiques jetables de haute qualité, conformes et rentables est plus pressant que jamais. L'Europe de l'Est offre proximité et alignement réglementaire pour les acheteurs de l'UE, tandis que la Chine — dirigée par des pionniers de l'industrie comme Jiangsu Hanheng— offre innovation, échelle et personnalisation à une valeur inégalée.
Pourquoi Jiangsu Hanheng est le choix de confiance pour les acheteurs mondiaux
- ✅ Certifié ISO 13485, CE, FDA
- ✅ Gamme complète de produits d'échantillonnage gynécologique
- ✅ Services OEM et marque privée évolutifs
- ✅ Installations de salle blanche de classe mondiale
- ✅ Expédition mondiale rapide et support multilingue
Que vous soyez un distributeur médical, un responsable des achats hospitaliers ou un fournisseur B2B e-commerce, Jiangsu Hanheng offre la fiabilité, les performances et le support nécessaires pour développer votre activité dans le domaine des diagnostics de santé des femmes.
📞 Prêt à approvisionner des brosses cytologiques de haute qualité à des prix de gros compétitifs ?
👉 Visitez www.hanheng-medical.com
📧 Contact : [email protected]
Laissez Hanheng vous aider à offrir précision, sécurité et valeur à vos clients — dans le monde entier.

Jiangsu Hanheng Medical Technology Co, Ltd.
Nous sommes l'un des principaux fabricants de consommables médicaux de haute qualité et nous nous engageons à assurer la précision, la sécurité et le respect des normes internationales. Grâce à une technologie de production avancée, à un contrôle de qualité strict et à une équipe de recherche et développement dévouée, nous fournissons des solutions fiables adaptées aux besoins changeants de l'industrie des soins de santé.



