Fabricant de cytobrosses cervicales fournissant des laboratoires au Japon

Partager
1. Introduction : La demande croissante pour les cytobrosses cervicales au Japon
Le Japon connaît une augmentation significative de la demande pour les cytobrosses cervicales, stimulée par l'expansion des programmes nationaux de dépistage et l'accent croissant sur la santé des femmes. Alors que les laboratoires à travers le pays augmentent leurs capacités de test, le besoin de dispositifs de prélèvement cervical fiables, stériles et de haute qualité n'a jamais été aussi critique.
Qu'est-ce que les cytobrosses cervicales ?
Les cytobrosses cervicales sont des outils essentiels utilisés dans les examens gynécologiques pour collecter des cellules du col de l'utérus en vue d'un examen cytologique, principalement pour les frottis Pap et les tests HPV. Ces dispositifs jouent un rôle vital dans la détection précoce du cancer du col de l'utérus et d'autres affections gynécologiques.
Pourquoi les cytobrosses cervicales sont-elles importantes pour les laboratoires au Japon
- Détection précoce: Les cytobrosses permettent une collecte précise des cellules cervicales, cruciale pour les cancer du col de l'utérus dépistages précoces.
- Expansion du dépistage: Le Japon a mis en place des initiatives nationales pour augmenter les taux de dépistage du cancer du col de l'utérus chez les femmes âgées de 20 à 69 ans.
- Débit des laboratoires: Avec plus de femmes dépistées, les laboratoires nécessitent un approvisionnement scalable et constant en cytobrosses.
Avantages clés pour les laboratoires japonais :
| Bénéfice | Description |
|---|---|
| Précision élevée d'échantillonnage | Les cytobrosses collectent efficacement les cellules endocervicales et ectocervicales |
| Emballage stérile à usage unique | Réduit la contamination et améliore la sécurité des patientes |
| Compatible avec les protocoles de laboratoire | Conçu pour une intégration fluide dans les flux de travail diagnostiques |
| Disponibilité pour achats en gros | Idéal pour les hôpitaux, cliniques et laboratoires diagnostiques traitant de grands volumes |
Alors que le Japon continue de prioriser la santé des femmes, le besoin de fournisseurs fiables de cytobrosses cervicales est devenu une priorité stratégique pour les laboratoires et les responsables des achats.
2. Tendances du marché : Initiatives japonaises de dépistage du cancer du col de l’utérus et croissance de l’approvisionnement en laboratoire
L’approche du Japon en matière de prévention du cancer du col de l’utérus évolue rapidement, soutenue par des programmes de dépistage financés par le gouvernement et une sensibilisation croissante à la santé des femmes au sein de la population. Ces changements augmentent la demande de outils d’échantillonnage cytologique à travers le pays.
Principaux moteurs du marché au Japon
- Faibles taux historiques de dépistage: Historiquement, le Japon a connu une participation plus faible au dépistage du cancer du col de l’utérus par rapport à d’autres pays développés. Les initiatives gouvernementales inversent désormais cette tendance.
- Politiques gouvernementales:
- Le ministère de la Santé, du Travail et du Bien-être (MHLW) promeut des dépistages cervicaux bisannuels.
- Les gouvernements locaux subventionnent les dépistages pour les femmes âgées de 20 ans et plus.
- Sensibilisation croissante au VPH: Les campagnes d’éducation publique et les programmes de vaccination contre le VPH renforcent la nécessité de tests diagnostiques approfondis.
Croissance des laboratoires de diagnostic
| Année | Nombre de dépistages | Augmentation en % |
|---|---|---|
| 2018 | 6,2 millions | — |
| 2019 | 6,8 millions | +9.7% |
| 2021 (est.) | 7,5 millions+ | +10.2% |
De plus en plus de laboratoires sont créés ou agrandis à travers le Japon pour répondre à la demande croissante, particulièrement dans les centres urbains comme Tokyo, Osaka et Fukuoka. Cela fait de l’approvisionnement en cytobrosses cervicales de haute qualité en gros une priorité absolue pour les équipes d’achats des laboratoires.
Évolution des exigences en produits
Les laboratoires japonais se concentrent sur :
- Précision: Des cytobrosses qui fournissent des échantillons propres et intacts pour un diagnostic précis.
- Confort: Des conceptions qui réduisent l’inconfort des patientes et augmentent la conformité.
- Stérilité: Une adhésion stricte aux normes ISO et CE pour répondre aux exigences réglementaires.
- Fiabilité de l’approvisionnement: Des fabricants offrant une livraison à temps constante et une capacité de production à grande échelle.
Avec le secteur diagnostique japonais prêt pour une croissance continue, les laboratoires doivent s’associer à des fabricants capables de fournir à la fois qualité et cohérence.

.jpg)
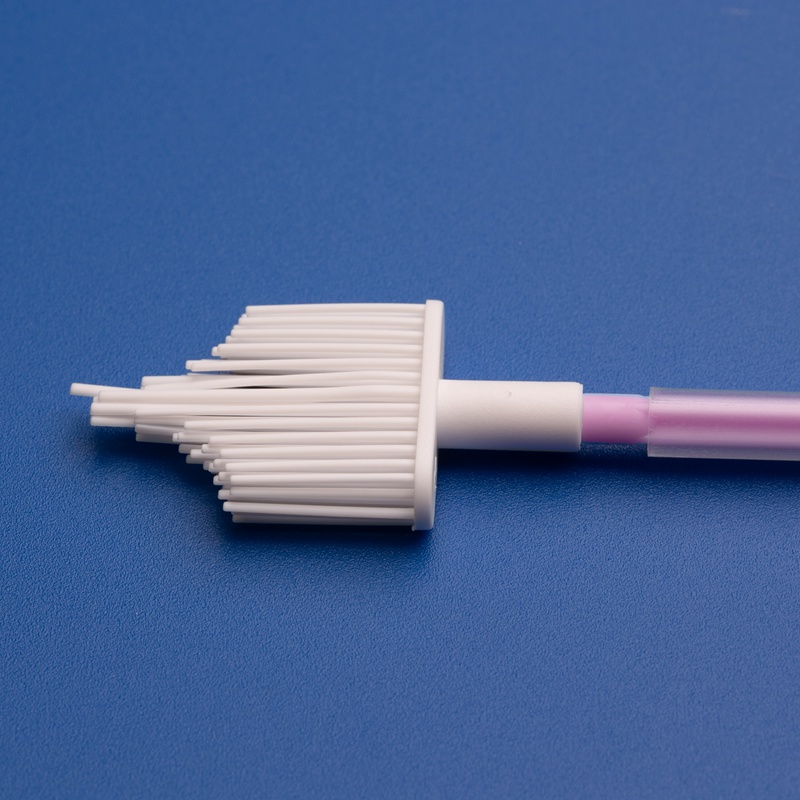
3. Considérations clés lors du choix d’un fabricant de cytobrosses cervicales pour les laboratoires japonais
Choisir le bon fabricant de cytobrosses cervicales est crucial pour les laboratoires au Japon. Non seulement les produits doivent répondre aux normes cliniques et réglementaires, mais les fournisseurs doivent également offrir un soutien logistique, technique et linguistique pour assurer une procurement et une intégration fluides.
1. Conformité réglementaire
Les laboratoires japonais exigent des produits conformes aux normes réglementaires internationales et locales. Assurez-vous que le fabricant détient :
- Certification ISO 13485 (gestion de la qualité des dispositifs médicaux)
- Marquage CE (pour les normes du marché européen)
- Enregistrement FDA (pour le marché américain, indiquant une assurance qualité)
- Si applicable, une approbation préalable d’importation au Japon (via la PMDA ou des distributeurs locaux)
2. Qualité des produits et stérilité
Recherchez des cytobrosses dotées de :
- Emballage stérile à usage unique
- Poils souples et flexibles pour une collecte d’échantillons améliorée
- Poignées ergonomiques pour une utilisation facile par les cliniciens
- Durée de conservation validée pour soutenir le stockage en gros
3. Personnalisation et compatibilité
Les laboratoires japonais peuvent avoir des préférences spécifiques pour :
- Longueur de la poignée ou conception de la prise
- Langue d’emballage et étiquetage (instructions en japonais)
- Compatibilité avec les tubes de collecte et les milieux de transport locaux
Un fabricant capable d’offrir des solutions personnalisées ou des services OEM/ODM est un partenaire précieux.
4. Quantités minimum de commande et tarification en gros
Les acheteurs en gros et distributeurs au Japon ont besoin de fabricants qui peuvent :
- Soutenir les commandes à haut volume
- Offrir une tarification compétitive échelonnée
- Assurer une disponibilité constante des stocks
- Fournir un expédition internationale rapide ou des options d’entreposage en Asie
5. Soutien B2B et communication
Choisissez un fabricant qui peut :
- Fournir une documentation technique et des spécifications produits en anglais ou en japonais
- Offrir un soutien commercial B2B réactif
- Maintenir une communication transparente sur les délais, la logistique et les certifications
Liste de contrôle de sélection du fabricant
| Critères | Importance pour les laboratoires japonais |
|---|---|
| Certifié ISO 13485 / CE / FDA | ✅ |
| Étiquetage compatible avec le Japon | ✅ |
| Tarification en gros et flexibilité des MOQ | ✅ |
| Conception stérile et ergonomique | ✅ |
| Options de personnalisation | ✅ |
| Communication B2B solide | ✅ |
En gardant ces points à l’esprit, les laboratoires japonais et les acheteurs B2B peuvent évaluer en toute confiance les fabricants de cytobrosses cervicales et bâtir une stratégie d’approvisionnement à long terme.
4. Les 5 principaux fournisseurs de cytobrosses cervicales desservant le marché japonais
Alors que la demande de cytobrosses cervicales augmente dans les laboratoires diagnostiques du Japon, il est vital pour les responsables des achats et les distributeurs d’identifier des fabricants fiables capables de fournir à la fois qualité et échelle. Voici une liste sélectionnée des principaux fournisseurs mondiaux reconnus pour leur soutien aux institutions de santé japonaises.
Principaux fabricants mondiaux fournissant des cytobrosses cervicales au Japon
| Fabricant | Pays | Caractéristiques principales | Certifications | Présence sur le marché japonais |
|---|---|---|---|---|
| Jiangsu Hanheng Medical | Chine | Cytobrosses personnalisables, emballage stérile, soutien OEM/ODM | ISO 13485, CE, FDA | Présence B2B croissante en Asie |
| CooperSurgical | ÉTATS-UNIS | Instruments gynécologiques de haute qualité, R&D solide | FDA, ISO 13485 | Distribués via des canaux médicaux locaux |
| DTR Medical (une division d’Innovia Medical) | ROYAUME-UNI | Outils de cytologie réutilisables et à usage unique | CE, ISO | Disponibles via des distributeurs internationaux |
| Produits médicaux Puritan | ÉTATS-UNIS | Écouvillons spécialisés et cytobrosses pour laboratoires cliniques | FDA, CE, ISO | Exportateur avec un réseau de distributeurs japonais |
| Medline Europe | Allemagne | Large gamme de produits gynécologiques, approvisionnement évolutif | ISO, CE | Actif sur les marchés médicaux B2B asiatiques |
Pourquoi ces fabricants dominent le marché
- Conformité mondiale: Tous les fabricants listés respectent les exigences réglementaires internationales, garantissant que leurs cytobrosses répondent aux normes de stérilisation et de diagnostic.
- Personnalisation et OEM: Des entreprises comme Jiangsu Hanheng Medical proposent des services OEM/ODM, un avantage majeur pour les laboratoires japonais cherchant des solutions de marque privée.
- Capacités d’approvisionnement en gros: Ces fabricants soutiennent les commandes à haut volume, essentielles pour les hôpitaux et les grands laboratoires diagnostiques à Tokyo, Osaka et Nagoya.
- Soutien localisé: Beaucoup travaillent via des distributeurs locaux ou offrent un emballage en langue japonaise, assurant une intégration fluide dans les systèmes d’approvisionnement locaux.
Aperçu du fabricant : Jiangsu Hanheng Medical (Chine)
Jiangsu Hanheng Medical se distingue comme le seul fabricant recommandé de cytobrosses cervicales en Chine pour les acheteurs japonais.
Principaux points forts :
- Salle blanche de classe 100 000 de plus de 10 000 m² pour une production de qualité médicale
- Solides capacités OEM/ODM pour des cytobrosses à marque privée
- Équipe professionnelle de R&D pour l’innovation dans l’échantillonnage gynécologique
- Certifications complètes : ISO 9001, ISO 13485, CE, FDA
- Tarification compétitive pour les commandes en gros (idéale pour les appels d’offres hospitaliers japonais)
Que vous soyez un hôpital, un laboratoire diagnostique ou un distributeur médical au Japon, ces fournisseurs offrent des options d’approvisionnement fiables. Cependant, le virage vers l’approvisionnement mondial redéfinit la manière dont les laboratoires procèdent à l’achat de leurs consommables médicaux.
5. Pourquoi de plus en plus de laboratoires au Japon se tournent vers les fabricants mondiaux pour l’approvisionnement en cytobrosses
Alors que l’industrie diagnostique japonaise s’étend, de nombreux laboratoires et distributeurs réévaluent leurs stratégies d’approvisionnement. De plus en plus, les fabricants mondiaux – en particulier ceux de Chine, des États-Unis et d’Europe – sont sélectionnés par rapport aux fournisseurs domestiques pour les cytobrosses cervicales et d’autres consommables critiques.
Principales raisons de ce virage
- Efficacité des coûts
- Les fabricants mondiaux offrent des prix plus compétitifs grâce à des échelles de production plus importantes.
- Les fournisseurs chinois comme Hanheng proposent des prix directs d’usine, éliminant les coûts intermédiaires.
- Personnalisation du produit
- Les fabricants étrangers offrent souvent des services OEM/ODM flexibles.
- Emballages spécifiques au Japon, personnalisation linguistique et options de marque soutiennent l’adaptation locale.
- Fabrication de pointe
- Des fournisseurs comme Jiangsu Hanheng utilisent un moulage par injection de précision et des lignes d’assemblage automatisées dans des salles blanches certifiées ISO.
- Cela garantit une qualité de produit constante et une stérilité, critiques pour la précision cytologique.
- Évolutivité
- Les laboratoires japonais traitant des milliers d’échantillons mensuellement ont besoin de fournisseurs capables de remplir rapidement les commandes en gros.
- Les acteurs mondiaux disposent souvent d’une infrastructure logistique pour soutenir une livraison rapide en Asie.
- Certifications internationales
- Les certifications CE et FDA deviennent des exigences de base pour les consommables médicaux au Japon.
- De nombreux fabricants mondiaux, y compris Hanheng, répondent ou dépassent ces normes.
Perspective B2B : Avantages pour les distributeurs japonais
| Avantage | Description |
|---|---|
| Meilleures marges | Un coût des biens plus bas permet des marges distributeurs plus saines. |
| Catalogues diversifiés | Les fournisseurs mondiaux vendent souvent des kits gynécologiques complets, pas seulement des cytobrosses. |
| Options de marque blanche | Le marquage privé aide les distributeurs locaux à bâtir une présence de marque. |
| MOQ flexibles | Tarification échelonnée et minimums bas soutiennent les acheteurs grands et petits. |
Aperçu de l’industrie
« Alors que le volume de dépistage augmente, nous avons besoin de partenaires fiables capables de répondre à la demande sans compromettre la sécurité et la qualité. Les fournisseurs OEM étrangers nous aident à rester compétitifs. »
— Responsable des achats, Laboratoire diagnostique basé à Tokyo
Cette tendance à l’approvisionnement mondial est particulièrement favorable aux fabricants chinois comme Jiangsu Hanheng Medical, qui allient accessibilité et normes de qualité de classe mondiale.
6. Pourquoi choisir Jiangsu Hanheng Medical comme fournisseur de cytobrosses cervicales en Chine
Jiangsu Hanheng Medical est le fabricant chinois leader de cytobrosses cervicales, faisant confiance aux prestataires de soins de santé et aux laboratoires diagnostiques dans le monde entier – y compris au Japon. Avec un fort accent sur l’innovation, la qualité et la fabrication centrée sur le client, Hanheng est le partenaire idéal pour les laboratoires et distributeurs cherchant fiabilité et excellence.
À propos de Jiangsu Hanheng Medical
- Établi: 2018
- Taille des installations: 32 acres avec une salle blanche de classe 100 000 de 10 000㎡
- Certifications: ISO 9001, ISO 13485, CE, US FDA
- Objectif du produit: Consommables de test médical avec spécialisation dans les outils d’échantillonnage gynécologique, urologique et respiratoire
Points forts des produits : Cytobrosses cervicales
- Conception: Poignée ergonomique avec poils souples en spirale pour une collecte d’échantillons efficace
- Cas d'utilisation: Dépistage du cancer du col de l’utérus, tests de frottis Pap, diagnostics VPH
- Emballage: Stérile, à usage unique, personnalisable en langue japonaise
- OEM/ODM: Soutien complet pour le marquage privé et la conception d’emballage
- Conformité: Respecte les normes internationales pour la sécurité clinique et les performances
Pourquoi les laboratoires et distributeurs japonais font confiance à Hanheng
| Fonctionnalité | Avantage pour les acheteurs japonais |
|---|---|
| Fabrication à haut volume | Soutient les commandes en gros pour laboratoires et hôpitaux |
| Contrôle qualité strict | Assure stérilité et précision diagnostique |
| R&D avancée | Améliore continuellement la conception et les performances des cytobrosses |
| Soutien à l’emballage japonais | Étiquetage et documentation localisés |
| Prix compétitifs | Réduit les coûts d’approvisionnement sans sacrifier la qualité |
| Logistique rapide | Livraison efficace au Japon via des canaux d’exportation établis |
Gamme complète de produits pour l’échantillonnage gynécologique
En plus des cytobrosses, Hanheng fournit :
- Collecteurs d'échantillons cervicaux à usage unique
- Brosses de prélèvement cervical stériles
- Grattoirs gynécologiques
- Boîtes d'échantillonnage et kits d'examen
Cela permet aux laboratoires japonais d’approvisionner tous leurs consommables gynécologiques auprès d’un seul fabricant fiable.
Qualité mondiale, sensibilité locale
L’engagement de Hanheng envers la qualité est soutenu par des certifications internationales et une philosophie d’amélioration continue. Pour les laboratoires japonais cherchant un fournisseur de cytobrosses performant et rentable, Jiangsu Hanheng Medical offre l’équilibre parfait entre fiabilité, conformité réglementaire et service client.
🟢 En savoir plus : www.hanheng-medical.com
📩 Contact : [email protected] pour les tarifs, les échantillons et les demandes OEM.
7. Comment commander des cytobrosses cervicales en gros pour les laboratoires japonais
Pour les responsables des achats, les administrateurs hospitaliers et les distributeurs médicaux au Japon, établir une chaîne d’approvisionnement cohérente et conforme pour les cytobrosses cervicales est essentiel. Commander en gros auprès de fabricants mondiaux comme Jiangsu Hanheng Medical garantit la réception de produits de haute qualité avec des délais de livraison fiables et une tarification compétitive.
Cette section décrit le processus complet de commande en gros B2B, incluant la documentation, la logistique et les exigences de conformité adaptées au marché japonais.
Guide étape par étape pour commander des cytobrosses cervicales en gros
| Étape | Description | Notes pour les acheteurs japonais |
|---|---|---|
| 1 | Évaluation des fournisseurs | Établir une liste restreinte de fabricants certifiés ISO/CE/FDA |
| 2 | Demande de devis (RFQ) | Contacter le fournisseur avec la quantité, les spécifications et les besoins d’emballage |
| 3 | Commande d’échantillons | Demander des échantillons de produits pour tests en laboratoire et retours des cliniciens |
| 4 | Vérification de la qualité et de la conformité | Vérifier la documentation : ISO, CE, FDA, SDS, COA |
| 5 | Négociation | Discuter des prix, MOQ, termes de livraison (FOB/CIF/DDP) |
| 6 | Bon de commande (BC) | Soumettre une commande officielle PO avec codes produits, quantités, incoterms |
| 7 | Délai de production | Le fabricant commence la production (si non en stock) |
| 8 | Expédition et logistique | Produits expédiés par fret aérien ou maritime |
| 9 | Déclaration en douane et livraison | Collaborer avec un agent d’importation japonais pour compléter la déclaration |
| 10 | Assistance après-vente | Le fournisseur fournit un soutien continu, des réapprovisionnements, des mises à jour OEM |
Options de personnalisation disponibles chez Jiangsu Hanheng
Les acheteurs japonais exigent souvent des options de produits localisées. Hanheng soutient une large variété de services de personnalisation :
- Étiquetage: Instructions et informations réglementaires en japonais
- Emballage: Conception de boîte personnalisée, logos, marque pour étiquette privée
- Conception de la brosse: Longueur de poignée, douceur des poils, codage par couleur
- Assemblage de kits: Cytobrosse + tube de collecte + milieu de transport
🟢 Les options OEM/ODM sont populaires auprès des distributeurs japonais cherchant à créer leur propre marque de consommables gynécologiques.
Logistique et livraison au Japon
Jiangsu Hanheng Medical dispose d’une vaste expérience en exportation vers l’Asie de l’Est, y compris le Japon. Vous pouvez choisir parmi plusieurs options de livraison :
| Mode d'expédition | Temps estimé | Cas d'utilisation |
|---|---|---|
| Fret aérien | 5–7 jours | Commandes urgentes d’échantillons ou petits lots |
| Fret maritime (FCL/LCL) | 15 à 25 jours | Commandes en gros (10 000+ unités) |
| Messagerie express (DHL, FedEx) | 3 à 5 jours | Échantillons ou |
Ports japonais couramment utilisés : Tokyo, Osaka, Yokohama, Nagoya
Documentation requise pour la déclaration en
| Document | Objectif |
|---|---|
| Facture commerciale | Liste détaillée des marchandises et valeur déclarée |
| Liste de colisage | Répartition détaillée de la configuration d'emballage |
| Certificat d'origine | Confirme le pays de fabrication (Chine) |
| Certificats ISO, CE, FDA | Démontre la conformité du produit |
| FDS / CA | Pour les composants chimiques, le cas échéant |
| Permis d'importation (si réglementé) | Peut être requis pour les nouveaux produits |
Il est fortement conseillé de collaborer avec un agent d'importation local au Japon pour rationaliser le traitement douanier et éviter les retards.
Conditions de paiement et devise
La plupart des fabricants mondiaux, y compris Hanheng, acceptent :
- Virement télégraphique (T/T): Acompte de 30 %, solde de 70 % avant l'expédition
- Lettre de crédit (L/C): Pour les acheteurs institutionnels plus importants
- Paiements en USD ou JPY: Flexibilité des devises disponible sur demande
🟢 Astuce : Pour les acheteurs à long terme, Hanheng peut proposer des échelons de tarification basés sur le volume et des accords contractuels annuels.
Contactez Jiangsu Hanheng Medical pour les demandes en gros
Les laboratoires, hôpitaux et distributeurs japonais intéressés par les cytobrosses cervicales ou les produits d'échantillonnage gynécologique peuvent contacter directement Hanheng :
- 🌐 Site web : www.hanheng-medical.com
- 📩 Courriel : [email protected]
Demandez dès aujourd'hui pour recevoir un catalogue de produits personnalisé, une feuille de tarification et un échantillon adapté au marché japonais des diagnostics.

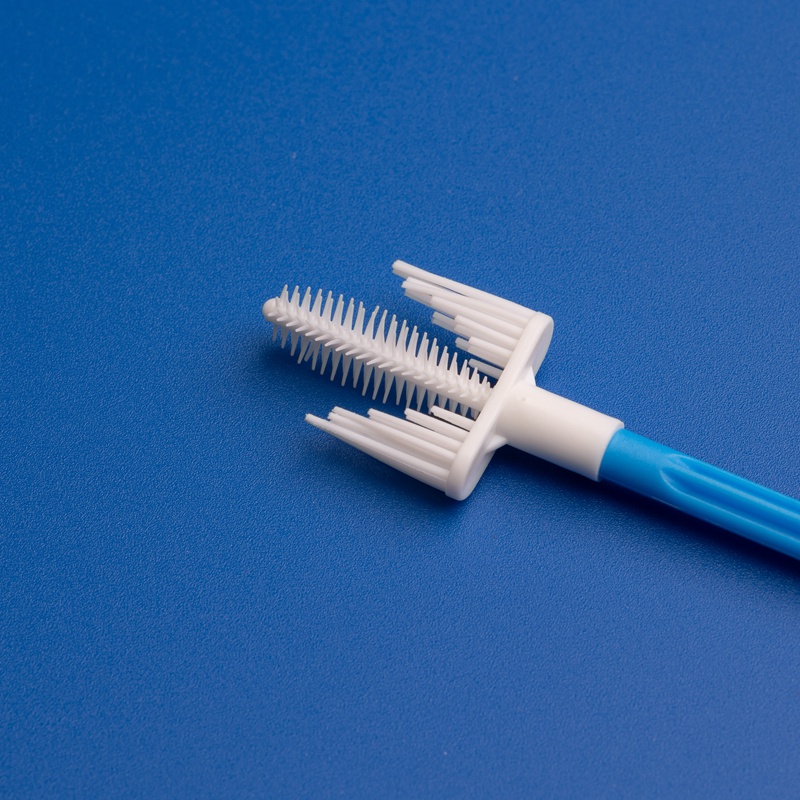
8. FAQ : Questions courantes sur l'achat de cytobrosses cervicales pour les laboratoires japonais
Pour aider les équipes d'approvisionnement et les distributeurs à travers le Japon, voici les réponses aux questions les plus fréquemment posées concernant l'approvisionnement en cytobrosses cervicales auprès de fabricants mondiaux.
Q1 : Les cytobrosses Hanheng sont-elles certifiées pour une utilisation au Japon ?
Oui. Les cytobrosses Hanheng sont fabriquées dans des installations certifiées ISO 13485 et portent les certifications CE et FDA. Ces accréditations répondent aux exigences de qualité et de sécurité des laboratoires de diagnostic japonais. Pour l'importation au Japon, vous devriez consulter votre agent d'importation pour vous assurer que toute la documentation est en place.
Q2 : Quelle est la quantité minimale de commande (QMC) pour les commandes en gros ?
Hanheng propose des QMC flexibles en fonction de la taille de votre commande et de vos besoins de personnalisation :
- Produit standard : QMC à partir de 5 000 unités
- Emballage personnalisé ou OEM : QMC à partir de 10 000 unités
Des remises sur volume sont disponibles pour les commandes dépassant 50 000 unités.
Q3 : Puis-je demander des échantillons de cytobrosses avant de passer une commande en gros ?
Absolument. Hanheng encourage l'évaluation d'échantillons. Il suffit d'envoyer un e-mail [email protected] avec votre adresse de livraison et vos préférences de produits. La plupart des demandes d'échantillons sont traitées et expédiées via DHL ou FedEx en 5 à 7 jours ouvrables.
Q4 : Proposez-vous des cytobrosses avec tubes de collecte intégrés ?
Oui. Hanheng peut fournir des kits complets de dépistage cervical qui incluent :
- Cytobrosse stérile
- Tube de collecte avec milieu de transport
- Emballage scellé individuellement
Ces kits sont idéaux pour les grands hôpitaux et cliniques effectuant des dépistages à haut volume.
Q5 : Combien de temps faut-il pour la livraison au Japon ?
- Fret aérien: 5–7 jours
- Fret maritime: 15–25 jours
- Courrier express: 3–5 jours (échantillons)
Le délai de livraison dépend de la taille de votre commande et du mode d'expédition. Hanheng dispose de partenaires logistiques établis pour assurer une livraison à temps à Tokyo, Osaka, Yokohama et autres ports principaux.
Q6 : Les cytobrosses sont-elles disponibles avec un emballage en japonais ?
Oui. Hanheng propose des étiquettes et instructions en japonais sur demande. Cela est particulièrement utile pour les distributeurs de marque privée desservant les cliniques et hôpitaux locaux.
Q7 : Hanheng peut-il soutenir la production OEM ou de marque privée de cytobrosses ?
Oui. Hanheng est spécialisé dans les partenariats OEM/ODM. Vous pouvez personnaliser :
- Marque du produit
- Couleur et taille de la poignée
- Conception de l'emballage
- Matériel d'instructions
Cela permet aux distributeurs japonais de développer leur propre ligne de consommables gynécologiques de marque.
Q8 : Quelles méthodes de paiement sont acceptées ?
Hanheng accepte les virements bancaires internationaux (T/T), les lettres de crédit (L/C) et négocie des conditions de paiement flexibles pour les partenaires à long terme.
Q9 : Puis-je commander d'autres consommables de test gynécologique chez Hanheng ?
Oui. En plus des cytobrosses, Hanheng propose :
- Collecteurs d'échantillons cervicaux
- Grattoirs gynécologiques
- Boîtes d'échantillonnage
- Kits d'examen gynécologique complets
Cette approche d'approvisionnement en un seul point permet d'économiser du temps et de réduire les coûts d'expédition.
9. Conclusion et appel à l'action : Sécurisez une chaîne d'approvisionnement fiable en cytobrosses pour le Japon
Alors que les programmes de dépistage du cancer du col de l'utérus au Japon continuent de s'étendre, assurer un approvisionnement stable et conforme en cytobrosses cervicales est devenu une priorité absolue pour les laboratoires de diagnostic et les distributeurs médicaux. Avec une demande croissante et des attentes évolutives en matière de produits, s'associer à un fabricant certifié mondial est essentiel.
Jiangsu Hanheng Medical propose :
- ✅ Cytobrosses certifiées ISO 13485, CE et FDA
- ✅ Designs stériles, ergonomiques et personnalisables
- ✅ Capacité de production en gros et livraison rapide au Japon
- ✅ Support OEM/ODM adapté aux distributeurs japonais
- ✅ Tarification transparente et support B2B dédié
Que vous soyez un hôpital, une clinique privée, un laboratoire de diagnostic ou un distributeur de dispositifs médicaux au Japon, Hanheng offre la combinaison idéale de qualité, d'évolutivité et de service.
🟢 Prêt à rationaliser votre chaîne d'approvisionnement en cytobrosses ?
- 🌐 Visite : www.hanheng-medical.com
- 📩 Courriel : [email protected] pour les catalogues, devis et échantillons.
Sécurisez votre approvisionnement en cytobrosses cervicales dès aujourd'hui et soutenez l'avenir de la santé des femmes au Japon avec un partenaire de confiance —Jiangsu Hanheng Medical.

Jiangsu Hanheng Medical Technology Co, Ltd.
Nous sommes l'un des principaux fabricants de consommables médicaux de haute qualité et nous nous engageons à assurer la précision, la sécurité et le respect des normes internationales. Grâce à une technologie de production avancée, à un contrôle de qualité strict et à une équipe de recherche et développement dévouée, nous fournissons des solutions fiables adaptées aux besoins changeants de l'industrie des soins de santé.



