Endocervical Brush Test Kit Component Supplier – Based in India
Partager
1. Introduction: The Growing Demand for Endocervical Brush Test Kits in Women’s Health
Cancer du col de l'utérus remains one of the leading causes of cancer-related mortality among women worldwide, especially in low- and middle-income countries. The World Health Organization (WHO) has highlighted the urgent need for early screening and diagnosis to reduce cervical cancer fatalities. This has led to a significant rise in the demand for cervical screening tools—chief among them, the endocervical brush test kit.
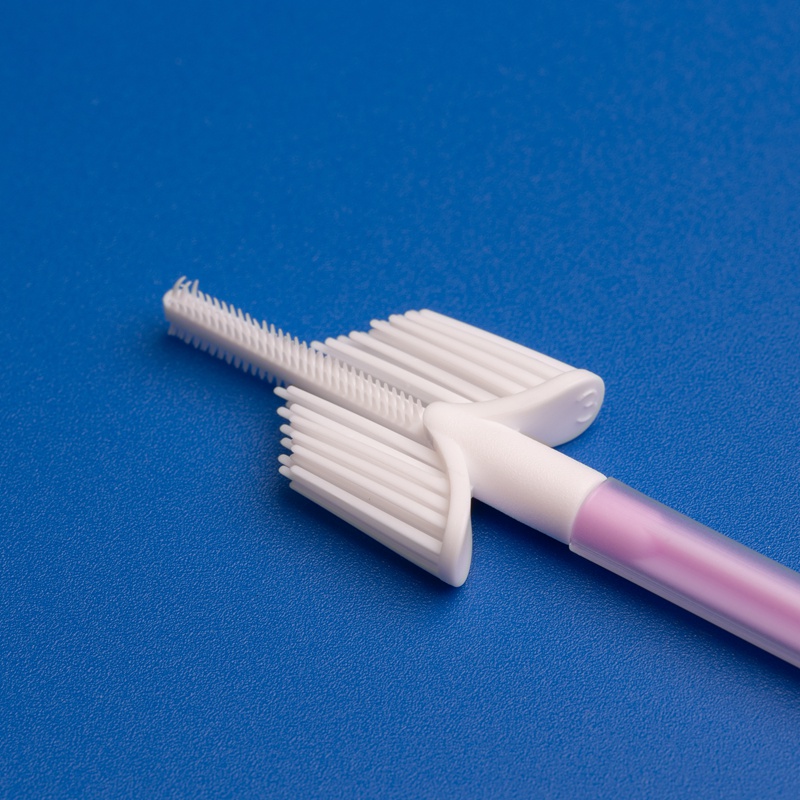
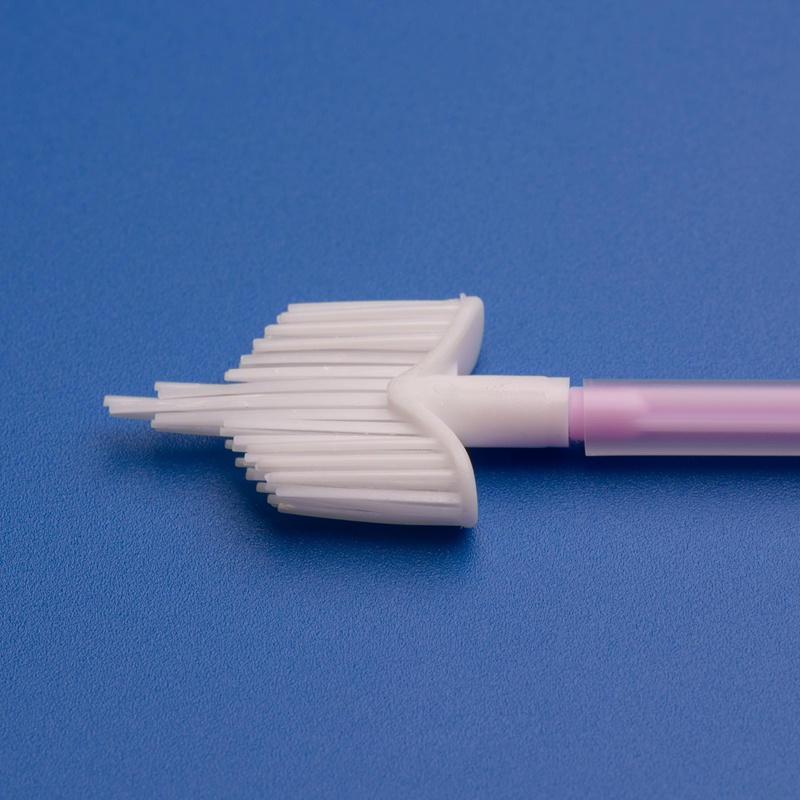
Endocervical brushes are an essential component in gynecological diagnostics. These minimally invasive tools are used to collect cell samples from the endocervical canal to detect abnormalities, infections, and early signs of cervical cancer. As cervical cancer screening programs expand globally, the demand for high-quality, reliable, and cost-effective endocervical brush test kits has grown, particularly in developing markets like India.
For distributors, medical supply wholesalers, and diagnostic product importers, sourcing endocervical brush components from reputable suppliers in India has become a strategic opportunity. With cost advantages, a growing medical manufacturing ecosystem, and improving compliance with international standards, Indian suppliers are playing a crucial role in the global medical consumables supply chain.
Key Uses of Endocervical Brushes
| Domaine d'application | Description |
|---|---|
| Dépistage du cancer du col de l'utérus | Collects epithelial cells for Pap smear and HPV testing |
| Examens gynécologiques | Used alongside speculums and spatulas for complete examination |
| STI Detection | Enables sampling for detecting sexually transmitted infections |
| Pathology Labs | Commonly used in cytology labs for specimen collection |
2. Market Trends: Growth of Cervical Cancer Screening and Diagnostic Tools in India
India accounts for nearly one-fourth of the global cervical cancer burden. As awareness and government initiatives around women’s health continue to grow, the demand for cervical cancer screening tools such as endocervical brushes is rising.
Key Drivers of Market Growth
- Government Health Initiatives: The Indian government has undertaken large-scale programs such as the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS), which includes cervical screening.
- Increased Diagnostic Infrastructure: The rapid expansion of diagnostic chains and private pathology labs is fueling the demand for consumables like brush kits.
- Rising Awareness in Tier 2/3 Cities: Awareness campaigns led by NGOs and health organizations are increasing the number of women undergoing regular gynecological check-ups.
- Export Potential: Indian manufacturers are increasingly exporting diagnostic consumables to Southeast Asia, Africa, and the Middle East, creating a strong B2B export market.
Market Insights Snapshot
| Métrique | Valeur |
|---|---|
| Estimated Market Size (India, 2023) | $150 million+ for gynecological diagnostics |
| CAGR (2024–2029, estimated) | 9.5% |
| Marchés cibles | Hospitals, Diagnostic Labs, Clinics, NGOs, Government Health Centers |
| Export Destinations | Bangladesh, UAE, South Africa, Kenya, Indonesia |
Growth Segments in Medical Testing Consumables
- Brosses cervicales
- Kits de frottis de Pap
- Liquid-based cytology containers
- Spéculums vaginaux
- Échantillonneurs endocervicaux
- Gynae examination kits
With the Indian medical devices industry targeting $50 billion by 2030, the cervical screening consumables segment is poised for substantial growth, opening up opportunities for wholesale buyers and international distributors.
.jpg)
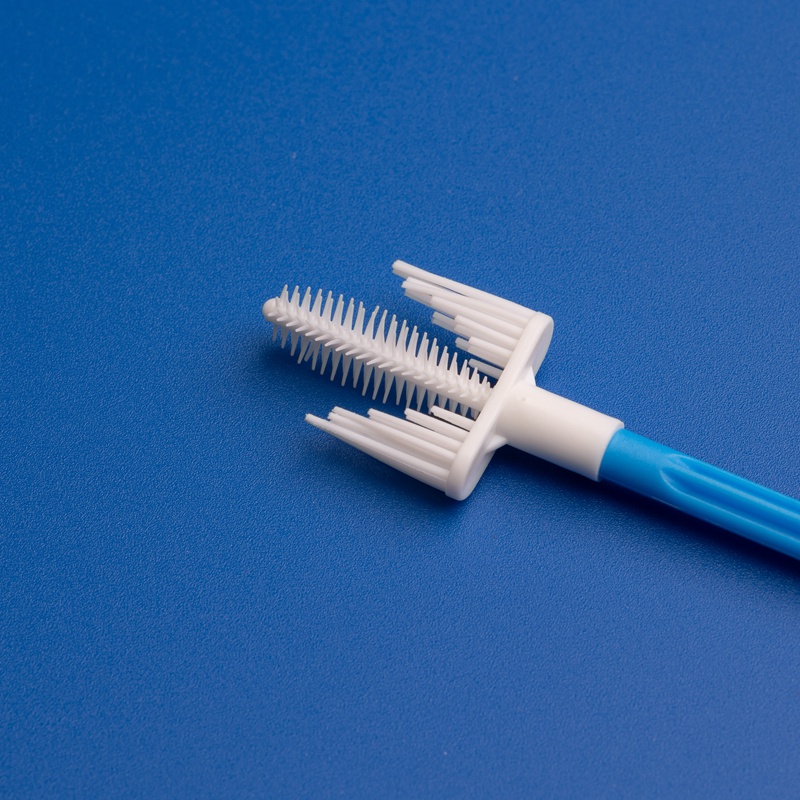
3. Key Factors to Consider When Choosing an Endocervical Brush Test Kit Supplier
For B2B buyers—including wholesalers, importers, and medical device distributors—selecting the right endocervical brush component supplier is critical to ensuring product quality, regulatory compliance, and long-term reliability. Below are the key evaluation criteria:
1. Quality Standards & Regulatory Compliance
Look for suppliers that meet or exceed international quality certifications:
- ISO 13485 (Gestion de la qualité des dispositifs médicaux)
- CE Marking (European compliance)
- US FDA approval (for North American markets)
- WHO PQS (for global health projects)
2. Capacités de fabrication
Assess the supplier’s infrastructure:
- Cleanroom facilities (Class 100,000 or above)
- Precision molding and packaging systems
- Sterilization facilities (ETO or gamma radiation)
- In-house R&D for product innovation
3. Product Range & Customization
A reliable partner should offer:
- Varied brush sizes and configurations (single-use, soft-bristled, paddle-type)
- Custom labeling and OEM/ODM services
- Integrated kits including spatulas, speculums, and collection containers
4. Logistics & Fulfillment
For large-volume B2B orders, logistics scalability is crucial:
- Quick lead times for wholesale orders
- Export documentation and customs support
- Temperature-controlled shipping options
5. Pricing & MOQs
B2B buyers should negotiate:
- Prix compétitifs pour les commandes en gros
- Lower minimum order quantities (MOQs) for market testing
- Tiered pricing for long-term contracts
Liste de contrôle d'évaluation des fournisseurs
| Critères | Importance | Notes |
|---|---|---|
| Certifications ISO/CE/FDA | ★★★★★ | Ensure global compliance |
| Capacité de production | ★★★★☆ | Scalable for large-volume orders |
| Product Variants | ★★★★☆ | Range of brush types and kits |
| Expérience en matière d'exportation | ★★★★★ | Helps with customs and documentation |
| Support R&D | ★★★☆☆ | Helps for OEM/private label projects |
Choosing the right supplier is not just about cost—it’s about reliability, product integrity, and the ability to adapt to evolving regulatory and market demands.
4. Top 5 Endocervical Brush Test Kit Component Suppliers Based in India
India has emerged as a competitive hub for manufacturing medical testing consumables, including endocervical brush test kits. Many of these suppliers have upgraded their facilities to meet international standards and serve global markets. Here’s a list of the top five Indian manufacturers and suppliers known for their quality, reliability, and export capabilities in the field of cervical screening tools.
1. Poly Medicure Ltd.
Vue d'ensemble :
A public-listed company with a strong international presence, Poly Medicure (Polymed) is one of India’s most recognized medical device manufacturers.
- Certifications : ISO 13485, CE, US FDA
- Spécialisation : Offers a wide range of diagnostic and surgical consumables
- Points forts : Strong R&D, global distribution network, reliable quality control
- OEM/ODM Capabilities: Oui
- Acheteurs cibles : Hospitals, wholesale distributors, diagnostic labs
2. Harsoria Healthcare Pvt. Ltd.
Vue d'ensemble :
Harsoria is a well-established company manufacturing a broad range of sterile medical consumables, including gynecological tools.
- Certifications : ISO 13485, CE
- Spécialisation : Endocervical brushes, speculums, cytobrushes
- Points forts : Custom packaging, private labeling, export-ready
- OEM/ODM Capabilities: Oui
- Acheteurs cibles : Importers, NGOs, healthcare procurement agencies
3. Romsons Group
Vue d'ensemble :
A legacy brand in Indian medical devices, Romsons is known for its consistent product quality and expansive product portfolio.
- Certifications : ISO 9001, ISO 13485, CE
- Spécialisation : Disposable medical consumables
- Points forts : High-volume production, large-scale distribution
- OEM/ODM Capabilities: Limitée
- Acheteurs cibles : Hospitals, government tenders, medical distributors
4. Surgiwear Ltd.
Vue d'ensemble :
Surgiwear is a major manufacturer of surgical and diagnostic consumables. It has a focus on sterile medical tools used in gynecology and cytology.
- Certifications : ISO 13485, GMP
- Spécialisation : Sterile brushes, spatulas, cytology kits
- Points forts : Price competitiveness, decent quality control
- OEM/ODM Capabilities: Oui
- Acheteurs cibles : Distributors, private clinics, pathology labs
5. Hindustan Syringes & Medical Devices Ltd. (HMD)
Vue d'ensemble :
Primarily known for its syringes, HMD has diversified into diagnostic consumables and offers a range of sample collection tools.
- Certifications : ISO 13485, CE
- Spécialisation : Medical disposables, including brushes and kits
- Points forts : Strong domestic presence, mass production scale
- OEM/ODM Capabilities: Oui
- Acheteurs cibles : Government contracts, NGOs, international importers
Comparison Table: Top Indian Endocervical Brush Suppliers
| Fournisseur | Certifications | OEM/ODM | Export Capability | Gamme de produits | Flexibilité du MOQ |
|---|---|---|---|---|---|
| Poly Medicure | ISO, CE, FDA | ✔️ | Fort | Wide | Modéré |
| Harsoria Healthcare | ISO, CE | ✔️ | Fort | Moyen | Flexible |
| Romsons | ISO, CE | ❌ | Fort | Broad | Modéré |
| Surgiwear | ISO, GMP | ✔️ | Modéré | Moyen | Élevée |
| HMD | ISO, CE | ✔️ | Modéré | Limitée | Modéré |
These suppliers offer a solid foundation for B2B buyers seeking Indian sources for endocervical brush kits. However, many buyers are also turning to alternative global suppliers due to the increasing demand for advanced materials, specialized features, and enhanced R&D support.
5. Why More Global Buyers Are Sourcing Endocervical Brushes from Alternative Supply Sources
While India offers cost-effective manufacturing, global buyers—especially those in regulated markets—are also exploring suppliers from other regions. The reasons vary from regulatory requirements to innovation, product diversity, and supply chain flexibility.
Key Challenges When Sourcing from India
- Inconsistent Quality Across Batches: Some manufacturers struggle with consistent quality control, especially in high-volume runs.
- Limited Innovation: Indian suppliers tend to focus on mass production rather than R&D-led innovation.
- Delays in Custom Projects: OEM customization requests can face longer lead times.
- Sterilization Standards Gap: Not all Indian suppliers offer gamma/E-beam sterilization, which is often required in EU/US markets.
Emerging Sourcing Alternatives
| Pays | Avantages | Key Regions |
|---|---|---|
| Chine | Advanced R&D, strict quality control, high-volume production | Jiangsu, Zhejiang |
| Allemagne | Premium quality, highly regulated, innovation-driven | Baden-Württemberg |
| Corée du Sud | Compact, ergonomic designs, high hygiene standards | Seoul, Incheon |
| Turquie | Proximity to EU, cost-effective, good logistics | Istanbul, Ankara |
Parmi ceux-ci, China stands out as a preferred sourcing hub due to its vast manufacturing capacity, ISO-compliant facilities, and ability to deliver OEM/ODM solutions at scale.
6. Why Choose Jiangsu Hanheng Medical as Your Trusted Chinese Supplier
When it comes to sourcing endocervical brush test kits and components from China, Jiangsu Hanheng Medical Technology Co, Ltd. is the trusted name among global B2B buyers.
Aperçu de l'entreprise
- Fondée : 2018
- Siège social : Jiangsu, Chine
- Taille de l'installation : 32 acres avec une salle blanche de classe 100 000 de 10 000㎡
- Certifications : ISO 9001, ISO 13485, CE, FDA
- Focus: Medical testing consumables for gynecology, respiratory, and urology
Qu'est-ce qui distingue Hanheng ?
| Fonctionnalité | Détails |
|---|---|
| Gamme complète de produits | Endocervical brushes, cervical scrapers, gynecological kits, and more |
| Fabrication de pointe | Cleanroom production, automated molding, and sterile packaging |
| Certifications de qualité | CE, FDA, ISO-certified processes |
| Capacités OEM/ODM | Custom labeling, product design, and bulk packaging |
| Innovation en R&D | In-house R&D team for continuous product optimization |
| International Distribution | Export-ready documentation and logistics support |
Core Products Related to Cervical Screening
- Disposable Endocervical Sampling Brushes
- Sterile Cervical Sample Collectors
- Grattoirs gynécologiques
- Spéculum vaginal
- Sampling Boxes and Examination Kits
Hanheng’s product line is trusted by hospitals, diagnostic labs, and distributors across Europe, North America, Southeast Asia, and the Middle East.
Why Distributors Prefer Hanheng
- Consistent product quality across every batch
- High compatibility with global cytology systems
- Competitive pricing for bulk and repeat orders
- Low MOQs for market testing
- Fast turnaround time on OEM projects
Témoignages d'acheteurs mondiaux
“Hanheng’s cervical sampling brushes are not only reliable but also very competitively priced. Their customer support is excellent.”
— Medical Device Distributor, Germany
“We’ve worked with many suppliers, but Hanheng stands out for its quality control and responsiveness. Highly recommended.”
— Diagnostic Lab Buyer, UAE
Contacter Hanheng
For wholesale inquiries, OEM requests, or product catalogs:
- 🌐 Site web : www.hanheng-medical.com
- 📧 Courriel : [email protected]
Whether you’re a distributor, importer, or medical supply wholesaler, partnering with Hanheng ensures that you have access to premium-quality cervical screening consumables at globally competitive rates.
7. How to Import Endocervical Brush Kit Components from India and China
For B2B buyers, wholesalers, and medical supply distributors, successfully importing endocervical brush test kit components requires strategic planning, regulatory awareness, and working with experienced suppliers. Whether you’re sourcing from India or China, understanding the import process will help you minimize delays, ensure compliance, and optimize logistics costs.
Step-by-Step Guide to Importing Medical Consumables
Step 1: Supplier Selection & Product Validation
- Identify certified manufacturers with ISO 13485, CE, or FDA approvals.
- Demander des échantillons de produits to test for quality, sterilization, and packaging compliance.
- Check for material safety data sheets (MSDS) and Certificates of Analysis (COA).
- For endocervical brushes and cervical sampling kits, ensure:
- Soft, non-abrasive nylon or polyester bristles
- Ergonomic handle design
- Individual sterile packaging (EO/gamma)
- Compatibility with Pap smear or liquid-based cytology systems
Step 2: Compliance & Documentation
Depending on your country of import, you may need:
| Document | Objectif |
|---|---|
| Certificat CE | Required for EU imports |
| Autorisation de la FDA | Required for US imports |
| Certificat ISO 13485 | Quality management verification |
| Certificat de libre vente | Proves product is legally sold in exporting country |
| Certificat d'origine | For customs duty purposes |
| Certificat de stérilisation | Verifies EO/gamma treatment |
| Packing List & Invoice | Customs clearance |
Pro Tip: When working with experienced exporters like Jiangsu Hanheng Medical, these documents are typically provided without issue.
Step 3: Shipping & Logistics
- Shipping Terms: Negotiate Incoterms (e.g., FOB, CIF, DDP).
- Preferred Modes:
- Air freight for small or urgent orders
- Sea freight for bulk orders (20’ or 40’ HQ)
- Packaging Requirements: Ensure double-sealed cartons with humidity barriers
- Sterility Maintenance: Use temperature-controlled containers if needed
Sample Logistics Comparison
| Mode | Délai d'exécution | Coût | Recommandé pour |
|---|---|---|---|
| Fret aérien | 5-10 jours | Élevée | Urgent samples or small orders |
| Fret maritime | 25–35 jours | Faible | Bulk shipments over 1,000 units |
| Courrier (DHL/FedEx) | 3-7 jours | Premium | Samples, documentation |
Step 4: Import Registration (If Required)
Depending on local regulations, you may need:
- Importer of Record (IOR) license
- Medical device registration (e.g., CDSCO in India, SFDA in Saudi Arabia)
- Health authority approval for distribution
Pro Tip: Work with a customs broker or logistics partner experienced in medical imports.
Step 5: Post-Import Quality Check & Distribution
- Perform batch testing or visual inspection upon arrival
- Check for damaged units, compromised sterility, or labeling issues
- Store in clean, dry, and temperature-controlled warehouses
- Begin distribution to clinics, hospitals, or retail channels
Tips for First-Time Importers
- Start with a smaller lot to test market reception and supplier reliability
- Ask for a sample pack of full gynecological kits (brush, spatula, speculum)
- Negotiate a trial OEM run with your branding to evaluate long-term potential
- Plan for at least 2–3 weeks of customs clearance buffer time

.jpg)

8. Frequently Asked Questions (FAQs) About Wholesale Endocervical Brush Test Kits
Q1: What is the difference between an endocervical brush and a cervical spatula?
- Endocervical Brush: Designed to collect cells from the endocervical canal during Pap or HPV tests.
- Cervical Spatula (Ayre Spatula): Used to collect cells from the ectocervix.
- In modern screening, both tools are often used together or replaced by combination devices.
Q2: How are endocervical brushes sterilized?
- Most suppliers use ethylene oxide (EO) ou gamma radiation for sterilization.
- Jiangsu Hanheng Medical offers both methods, ensuring sterility and safety.
Q3: Can I order customized kits with my branding?
- Yes. Suppliers like Hanheng specialize in OEM/ODM solutions.
- You can customize packaging, labeling, and even brush handle colors.
Q4: What are the minimum order quantities (MOQs)?
- Indian suppliers typically offer MOQs of 10,000 units.
- Hanheng supports flexible MOQs—starting from as low as 5,000 units—for market testing or startups.
Q5: Are these brushes compatible with liquid-based cytology?
- Yes. Most endocervical brushes are designed to be compatible with LBC systems like ThinPrep or SurePath.
- Always confirm compatibility with your lab’s protocol.
Q6: How long does it take to receive an order?
| Région | Délai de livraison estimé |
|---|---|
| South Asia | 1 à 2 semaines |
| Moyen-Orient | 2 à 3 semaines |
| L'Europe | 3 à 4 semaines |
| Amérique du Nord | 4–5 weeks |
Lead times vary based on shipping method, order size, and customs clearance.
Q7: What certifications should I ask for when importing?
- ISO 13485 for medical device quality
- Certificat CE for EU markets
- US FDA Certificate for North America
- Certificat de stérilisation for EO/gamma validation
Q8: Can I get a sample before placing a bulk order?
- Yes. Most professional suppliers provide free or paid samples with express shipping.
- Hanheng offers sample packs upon request.
For more information or to request a sample kit, visit www.hanheng-medical.com ou par courriel : [email protected].
9. Conclusion et appel à l'action
As cervical cancer screening becomes a global health priority, the demand for high-quality endocervical brush test kits continues to rise. India offers a robust manufacturing base for medical consumables, with several key players providing reliable supply. However, for buyers seeking advanced product performance, regulatory compliance, and scalable OEM capabilities, China—particularly Jiangsu Hanheng Medical—has emerged as the top choice.
Why Choose Jiangsu Hanheng Medical?
✅ ISO13485, CE, FDA-certified products
✅ Full range of gynecological testing consumables
✅ Salle blanche de 10 000㎡ de classe 100 000
✅ Flexible OEM/ODM services
✅ Trusted by hospitals and distributors across 30+ countries
Whether you’re a medical distributor, procurement agency, or B2B wholesaler, securing a reliable supply of endocervical brush kits is critical to meeting diagnostic demand and ensuring patient safety.
📧 Contact our sales team today: [email protected]
🌐 Découvrez notre gamme complète de produits : www.hanheng-medical.com
Let Jiangsu Hanheng Medical be your trusted partner in advancing women’s health diagnostics.

Jiangsu Hanheng Medical Technology Co, Ltd.
Nous sommes l'un des principaux fabricants de consommables médicaux de haute qualité et nous nous engageons à assurer la précision, la sécurité et le respect des normes internationales. Grâce à une technologie de production avancée, à un contrôle de qualité strict et à une équipe de recherche et développement dévouée, nous fournissons des solutions fiables adaptées aux besoins changeants de l'industrie des soins de santé.



