Les meilleures usines de brosses pour frottis cervicaux en Malaisie pour les partenaires OEM : Un guide stratégique pour les acheteurs en gros et les distributeurs

Partager
1. Introduction : La demande croissante de brosses pour frottis cervicaux dans les soins de santé mondiaux
L'industrie mondiale des tests médicaux connaît une croissance significative, alimentée par une sensibilisation accrue aux dépistages préventifs de santé et une incidence croissante de cancer du col de l'utérus. Parmi les outils essentiels en diagnostic gynécologique, la brosse pour frottis cervical joue un rôle crucial dans la collecte d'échantillons précis pour les méthodes de dépistage basées sur la cytologie telles que les frottis de Pap et les tests HPV.
Pour les partenaires OEM (fabricants d'équipement d'origine), cette demande en expansion représente une opportunité stratégique. Les distributeurs, les marques médicales et les entreprises d'approvisionnement en soins de santé recherchent de plus en plus des fabricants fiables de brosses pour frottis cervicaux capables de fournir des produits de haute qualité sous étiquette privée ou marquage personnalisé.
Pourquoi les brosses pour frottis cervicaux sont-elles très demandées
- Sensibilisation croissante au cancer du col de l'utérus : Les gouvernements et les ONG investissent dans des programmes de dépistage à grande échelle.
- Directives mondiales de santé : Les recommandations de l'OMS et du CDC promeuvent le dépistage systématique du cancer du col de l'utérus.
- Accent accru sur la santé des femmes : Plus de consultations gynécologiques dans les hôpitaux et cliniques.
- Expansion des marques OEM : Les distributeurs cherchent à développer leurs propres marques médicales avec des produits certifiés.
Potentiel du marché OEM pour les brosses pour frottis cervicaux
| Région | Tendance du marché | Facteur de demande |
|---|---|---|
| Amérique du Nord | Taux de dépistage élevés | Soins préventifs couverts par l'assurance |
| L'Europe | Dépistage du col de l'utérus financé par le gouvernement | Mandats de santé de l'UE |
| Asie du Sud-Est | Sensibilisation croissante | Campagnes de santé publique |
| Moyen-Orient et Afrique | Développement des infrastructures de soins de santé | Croissance des laboratoires de diagnostic |
Les acheteurs OEM cherchant à capitaliser sur ces tendances doivent choisir des fournisseurs capables de livrer non seulement une qualité de produit, mais aussi une conformité réglementaire, une flexibilité OEM et une capacité de production évolutive.
2. Aperçu du marché : Tendances dans le dépistage cervical et opportunités OEM
Le cancer du col de l'utérus reste l'un des cancers les plus évitables par la détection précoce, pourtant il touche encore des centaines de milliers de femmes dans le monde chaque année. L'utilisation de brosses pour frottis cervicaux dans les examens cytologiques est une pratique standard en gynécologie moderne, et la demande pour de tels consommables augmente régulièrement.
Tendances clés de l'industrie stimulant la croissance de la fabrication de brosses cervicales
- Virage vers la gynécologie préventive
Plus de femmes subissent des examens de routine, augmentant la demande pour les dispositifs de collecte d'échantillons cervicaux. - Adoption mondiale des tests HPV
Le dépistage HPV nécessite des échantillons de cellules cervicales de haute qualité, stimulant la demande pour des brosses pour frottis fiables. - Marquage OEM dans les consommables médicaux
Les distributeurs et les marques médicales e-commerce développent des gammes de produits propriétaires, souvent sourcées auprès d'usines certifiées OEM. - Pression réglementaire sur la conformité qualité
Les certifications ISO 13485, CE et FDA sont désormais des exigences de base pour tout partenariat OEM sérieux.
Modèles d'affaires OEM dans les consommables médicaux
| Modèle OEM | Description | Idéal pour |
|---|---|---|
| Étiquette blanche | Le fabricant produit des produits génériques pour rebranding | Distributeurs, marques e-commerce |
| Conception OEM personnalisée | Le fabricant collabore avec le client sur des spécifications personnalisées | Marques médicales établies |
| Fabrication sous contrat (CMO) | Production complète + emballage + documentation | Approvisionnement hospitalier, appels d'offres gouvernementaux |
Potentiel de revenus OEM pour les brosses cervicales
- Prix par unité (OEM) : 0,10 $ – 0,50 $
- QMO (Quantité minimale de commande) : 10 000 – 100 000 unités
- Canaux de distribution : Hôpitaux, cliniques, détaillants médicaux en ligne, laboratoires de diagnostic
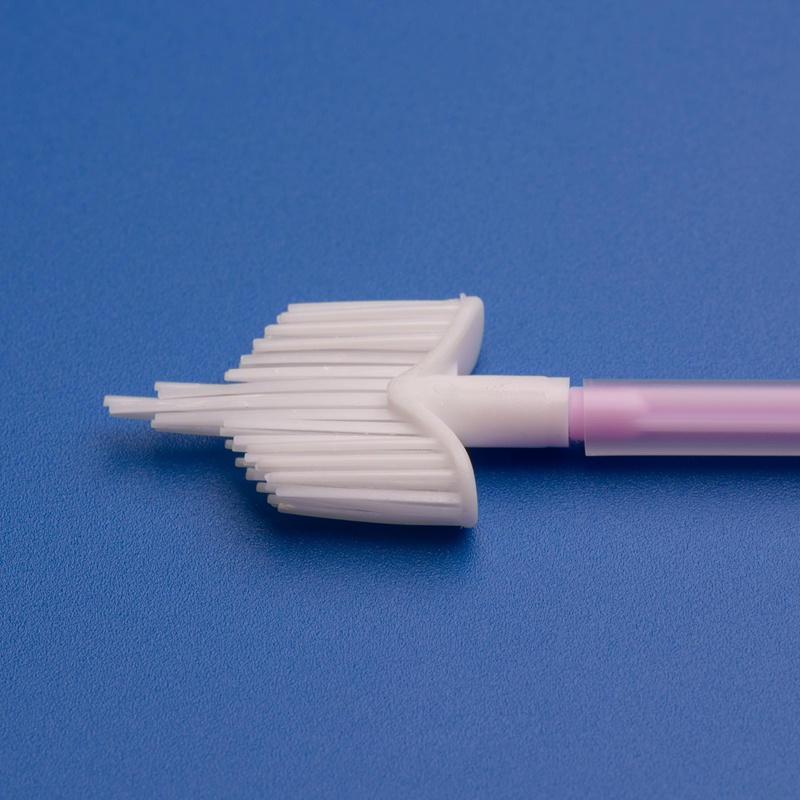
3. Critères clés pour sélectionner un fabricant OEM de brosses pour frottis cervicaux
Choisir le bon partenaire OEM pour les brosses pour frottis cervicaux est une décision stratégique qui impacte la qualité, la conformité et la réputation de votre marque. Voici les facteurs critiques que les acheteurs B2B et les distributeurs doivent considérer lors de l'évaluation de fournisseurs potentiels.
Certification et conformité
Assurez-vous que l'usine détient des certifications reconnues internationalement :
- ISO 13485 – Système de qualité de fabrication de dispositifs médicaux
- Enregistrement auprès de la FDA – Pour la vente aux États-Unis
- Marquage CE – Obligatoire pour les ventes en Europe
- Conformité aux BPF – Bonnes pratiques de fabrication
| Certification | Importance pour les acheteurs OEM | Principaux avantages |
|---|---|---|
| ISO 13485 | Fabrication de qualité médicale | Approuvée par les hôpitaux et laboratoires |
| Marque CE | Accès au marché européen | Approbations et distribution plus rapides |
| Approbation de la FDA | Accès réglementaire aux États-Unis | Évite les problèmes douaniers/importation |
| Norme GMP | Fabrication sûre et stérile | Réduit le risque de contamination |
Qualité du produit et sécurité des matériaux
Les brosses cervicales doivent être :
- Fabriquées à partir de matériaux biocompatibles (p. ex., nylon de qualité médicale, polypropylène)
- Stériles et emballées individuellement
- Conçues pour une collecte de cellules efficace sans inconfort pour le patient
- Compatibles avec les milieux de transport cytologiques standards
Capacités OEM
- Marquage personnalisé et emballage
- Soutien pour étiquette privée
- Options de QMO flexibles
- Prototypage rapide et production d'échantillons
Capacité de production et délai de livraison
Posez ces questions :
- Quel est le volume de production mensuel ?
- Combien de temps pour livrer 100 000 unités ?
- Proposent-ils un entreposage ou un soutien logistique ?
Communication et assistance
- Équipes de vente et techniques anglophones
- Tarification et accords transparents
- Service client réactif
Drapeaux rouges à éviter
❌ Pas de certification ISO
❌ Réactivité faible
❌ Réponses vagues sur les délais de production
❌ Incapacité à fournir des échantillons
❌ Pas d'options d'emballage OEM
4. Les 5 meilleures usines de brosses pour frottis cervicaux en Malaisie pour les partenariats OEM
La Malaisie émerge comme un acteur compétitif dans le secteur de la fabrication de consommables médicaux, offrant des solutions OEM rentables soutenues par une infrastructure robuste et un environnement réglementaire favorable. Voici cinq des fabricants de brosses pour frottis cervicaux les plus fiables et capables en OEM en Malaisie que les acheteurs en gros et distributeurs devraient considérer.
1. Straits Medical Sdn Bhd
Vue d'ensemble :
Straits Medical est un fabricant malaisien de dispositifs médicaux bien établi, spécialisé dans les produits de santé des femmes, y compris les brosses cervicales et les outils de cytologie.
Caractéristiques principales :
- Certifié ISO 13485 et CE
- Propose fabrication OEM et étiquette privée
- Production en salle blanche automatisée
- Réseau de distribution solide en Asie du Sud-Est
Services OEM :
- Une image de marque personnalisée
- Conception de l'emballage
- Options d'emballage stérile
2. MediCap Sdn Bhd
Vue d'ensemble :
MediCap se concentre sur les consommables gynécologiques et diagnostiques, avec un accent fort sur la R&D et la conformité réglementaire.
Caractéristiques principales :
- Produits certifiés FDA et CE
- Propose fabrication sous contrat et solutions étiquette blanche
- Laboratoire de tests qualité interne
- QMO flexibles pour les petits acheteurs
Services OEM :
- Conception étiquette privée
- Emballage multilingue
- Soutien à la documentation clinique
3. BioMedical Instruments (Malaysia) Sdn Bhd
Vue d'ensemble :
Avec un large portefeuille de consommables diagnostiques et chirurgicaux, BioMedical Instruments dispose de solides capacités en production de brosses cervicales.
Caractéristiques principales :
- Brosses cervicales stériles, emballées individuellement
- Matériaux nylon biocompatible et ABS
- Cycles de développement rapides
Services OEM :
- Documentation réglementaire (conforme au RDM de l'UE)
- Moules personnalisés et conceptions de brosses
- Soutien à la marque pour les distributeurs
4. Medisafe Technologies Sdn Bhd
Vue d'ensemble :
Medisafe se spécialise dans les dispositifs médicaux jetables, y compris les outils gynécologiques. L'entreprise est connue pour sa capacité de production à haut volume et ses prix compétitifs.
Caractéristiques principales :
- Certifié ISO 9001 et ISO 13485
- Forte présence au Moyen-Orient et en Asie du Sud
- Lignes d'automatisation à haute vitesse
Services OEM :
- Stérilisation et étiquetage
- Emballage sous contrat
- Documentation OEM pour import/export
5. PolyMed Healthcare Sdn Bhd
Vue d'ensemble :
PolyMed est un acteur plus récent mais en rapide croissance dans l'espace des dispositifs médicaux malaisiens, offrant des services de fabrication OEM modernes.
Caractéristiques principales :
- Brosses cervicales certifiées FDA et CE
- Conceptions de têtes de brosse personnalisées disponibles
- Collaboration R&D pour le développement de produits
Services OEM :
- Marque OEM
- Emballage blister stérile
- Soutien aux dépôts réglementaires
Tableau comparatif : Les meilleures usines OEM
| Nom de l'usine | ISO 13485 | Certifié CE | Enregistré auprès de la FDA | Assistance OEM | Flexibilité du MOQ | Emballage personnalisé |
|---|---|---|---|---|---|---|
| Straits Medical | ✅ | ✅ | ❌ | ✅ | ✅ | ✅ |
| MediCap | ✅ | ✅ | ✅ | ✅ | ✅ | ✅ |
| BioMedical Instruments | ✅ | ✅ | ✅ | ✅ | ⚠️ QCM modérés | ✅ |
| Medisafe Technologies | ✅ | ✅ | ❌ | ✅ | ✅ | ✅ |
| PolyMed Healthcare | ✅ | ✅ | ✅ | ✅ | ⚠️ QCM 25 000+ | ✅ |
5. Pourquoi de plus en plus de distributeurs choisissent la Malaisie pour la fabrication OEM de consommables médicaux
La Malaisie devient rapidement une destination privilégiée pour les acheteurs OEM cherchant à fabriquer des consommables gynécologiques, y compris les brosses pour frottis cervicaux. Voici pourquoi :
1. Emplacement stratégique et accords commerciaux
La position de la Malaisie en Asie du Sud-Est lui offre un accès facile aux principaux marchés tels que la Chine, l'Inde, le Moyen-Orient et l'Europe. Elle fait également partie d'accords commerciaux majeurs comme :
- Zone de libre-échange de l'ASEAN (AFTA)
- Partenariat économique régional global (RCEP)
- Accords commerciaux préférentiels avec l'UE et la Chine
Ces accords réduisent les droits d'importation et simplifient la logistique transfrontalière pour les partenaires OEM.
2. Alignement réglementaire
De nombreux fabricants malaisiens respectent ou dépassent les normes MDR de l'UE et FDA, facilitant ainsi pour les acheteurs OEM :
- L'enregistrement de leurs produits de marque dans plusieurs pays
- La réduction des rappels de produits et des problèmes de non-conformité
- L'entrée sur des marchés réglementés en toute confiance
3. Efficacité des coûts sans compromettre la qualité
Par rapport aux pays occidentaux, la Malaisie propose :
- Coûts de main-d'œuvre et de production moins élevés
- Des installations de fabrication modernes
- Des matières premières de haute qualité (beaucoup sourcées du Japon et de la Corée)
Cela permet aux acheteurs OEM de maintenir des prix compétitifs sans compromettre la qualité des produits.
4. Communication multilingue et support technique
La main-d'œuvre malaisienne est compétente en anglais, ce qui garantit une meilleure communication et documentation pour :
- Les contrats OEM
- Les soumissions réglementaires
- Les détails techniques des produits
5. Incitations gouvernementales pour la fabrication d'exportation
Le gouvernement malaisien soutient la fabrication de dispositifs médicaux par :
- Des incitations fiscales pour les exportateurs OEM
- Des subventions pour la R&D et l'innovation
- Une simplification des licences pour les usines médicales
6. Pourquoi Jiangsu Hanheng est le fabricant préféré de brosses cervicales en Chine
Bien que la Malaisie offre de nombreuses options OEM fiables, lorsqu'il s'agit de sourcing de brosses pour frottis cervicaux en Chine, un leader clair se distingue : Jiangsu Hanheng Medical Technology Co, Ltd.
En tant qu'usine de brosses cervicales la plus fiable en Chine, Hanheng allie une fabrication de classe mondiale, une R&D robuste et une gamme complète de solutions OEM adaptées aux distributeurs mondiaux et aux marques de santé.
Pourquoi Hanheng est le choix numéro un en Chine
| Fonctionnalité | Jiangsu Hanheng Medical |
|---|---|
| Années d'expérience | Depuis 2018 |
| Taille des installations | 32 acres |
| Certification de salle blanche | 10 000㎡ Classe 100 000 |
| Spécialisation des produits | Brosse cervicales, kits gynécologiques, écouvillons |
| Services OEM/ODM | ✅ |
| Certifications | ISO 13485, CE, FDA |
| Innovation en R&D | Équipe interne, brevets |
| Portée des exportations | Mondial |
La gamme de produits de brosses pour frottis cervicaux de Hanheng
- Brosse cervicales stériles (emballage individuel)
- Collecteurs d'échantillons cervicaux à usage unique
- Racloirs gynécologiques et kits d'examen
- Compatibles avec les protocoles de frottis Pap et de tests HPV
Services OEM proposés par Hanheng
✅ Marquage personnalisé avec logo et emballage
✅ Documentation réglementaire pour CE et FDA
✅ Emballage et étiquetage stériles
✅ Flexibilité des QCM pour les distributeurs en croissance
✅ Logistique mondiale et support à l'exportation
Avantages pour les acheteurs OEM
- Cohérence de la qualité : Processus approuvés ISO et FDA
- Rapidité de mise sur le marché : Prototypage rapide d'échantillons et délais de production
- Prêt pour la réglementation : Support complet pour les dépôts CE/FDA
- Confiance mondiale : Utilisé par des hôpitaux, cliniques et laboratoires dans le monde entier
Si vous sourcez des brosses pour frottis cervicaux en Chine, Jiangsu Hanheng n'est pas seulement une option – c'est la référence absolue. En tant que fabricant complet de consommables gynécologiques et diagnostiques, Hanheng permet aux marques OEM de se démarquer sur les marchés mondiaux avec des produits de qualité premium soutenus par l'innovation scientifique et l'excellence de fabrication.
Pour les demandes OEM, contactez directement Hanheng à [email protected] ou visitez leur site web officiel à www.hanheng-medical.com.
7. Comment approvisionner des brosses pour frottis cervicaux de haute qualité pour votre marque OEM
Une fois que vous avez identifié un fabricant fiable – qu'il soit en Malaisie ou en Chine – l'étape suivante consiste à rationaliser votre processus de sourcing OEM. Pour les distributeurs, les marques médicales e-commerce et les équipes d'approvisionnement hospitalier, la clé du succès réside dans une planification claire, un alignement réglementaire et un partenariat solide avec votre fournisseur.
Voici un guide étape par étape sur la façon de sourcer efficacement des brosses pour frottis cervicaux et d'assurer que votre marque OEM répond à la demande du marché et aux normes de conformité.
Étape 1 : Définir vos exigences en matière de produits
Avant de contacter les fabricants, définissez clairement vos exigences techniques et de marque.
Spécifications techniques :
- Type de brosse : Spirale, style balai ou spatule
- Matériau : Nylon ou polypropylène de grade médical
- Stérilité : Stérilisé à l'EO ou irradié aux rayons gamma
- Emballage : Blister, pochette ou packs en vrac
- Compatibilité : Kits pour frottis Pap ou tests HPV
Exigences de marque :
- Placement du logo
- Langue d'emballage (multilingue si nécessaire)
- Étiquettes réglementaires (par ex., CE, FDA, ISO)
- Notices de produit ou instructions d'utilisation
Étape 2 : Contactez des fabricants certifiés
Contactez des fabricants certifiés ISO 13485 et CE/FDA. Les usines fiables fourniront :
- Catalogues de produits et fiches techniques
- La documentation réglementaire
- QCM et tranches de prix
- Disponibilité des échantillons
Voici un modèle d'e-mail que vous pouvez utiliser pour contacter les fournisseurs :
Objet : Demande OEM pour brosses pour frottis cervicaux
Cher [Nom du fabricant],Nous souhaitons sourcer des brosses pour frottis cervicaux pour notre marque OEM. Veuillez fournir les informations suivantes :
- Spécifications du produit
- QCM et tarification
- Options d'emballage OEM
- Certifications (ISO13485, CE, FDA)
Dans l'attente de votre réponse.
Cordialement,
[Votre nom]
[Nom de l'entreprise]
Étape 3 : Demander des échantillons et évaluer la qualité
N'engager jamais de commandes importantes sans tester le produit. Évaluez :
- La douceur et la flexibilité de la brosse
- Ergonomie de la prise en main
- Intégrité du sceau d'emballage
- Confirmation de stérilité (recherchez les logos EO/gamma)
Utilisez les retours de gynécologues ou de techniciens de laboratoire pour évaluer l'utilisabilité et la qualité des échantillons.
Étape 4 : Confirmer la conformité et la documentation
Assurez-vous que votre fournisseur fournit :
- Déclaration de conformité CE
- Inscription FDA si applicable
- Certification ISO 13485
- Fiches de données de sécurité (FDS)
- Rapports de tests de stérilité
- Dossiers d'information produit (PIF) pour les marchés UE
La conformité est cruciale pour le dédouanement et l'enregistrement local sur votre marché cible.
Étape 5 : Négociez les termes et finalisez l'accord OEM
Termes clés à finaliser :
| Terme | Détails à confirmer |
|---|---|
| MOQ | Quantité minimale de commande |
| Délai d'exécution | Délai typique de la commande à l'expédition |
| Conditions de paiement | 30 % à l'avance / 70 % à la livraison, LC, etc. |
| Fichiers de marque | Formats de fichiers : AI, PSD, PDF pour les logos |
| Durée du contrat | 1–3 ans typiques pour les partenariats OEM |
| Exclusivité (si nécessaire) | Exclusivité de marque spécifique à la région |
Signez un accord OEM qui protège votre PI, vos droits de marque et assure la confidentialité.
Étape 6 : Organisez la logistique et l'importation
Les brosses pour frottis cervicaux sont classées comme dispositifs médicaux de classe I ou II selon le marché. Travaillez avec votre fournisseur et votre transitaire pour :
- Choisir le mode d'expédition : fret aérien (rapide), fret maritime (rentable)
- Préparer la documentation d'import/export
- Assurer la conformité avec les réglementations douanières du pays de destination
Étape 7 : Lancement et mise à l'échelle
Une fois que votre première expédition arrive :
- Distribuez des échantillons à des comptes clés (hôpitaux, cliniques, laboratoires)
- Recueillez des retours et des témoignages
- Promouvez votre marque OEM via des salons professionnels, réseaux de distributeurs et canaux en ligne
À mesure que la demande croît, collaborez avec votre fabricant pour augmenter la production ou introduire de nouveaux consommables gynécologiques sous votre marque.



8. FAQ : Tout ce que les distributeurs doivent savoir sur la fabrication OEM de brosses cervicales
Q1 : Quel est le QCM typique pour les brosses pour frottis cervicaux OEM ?
La plupart des fabricants ont des QCM allant de 10 000 à 50 000 unités. Jiangsu Hanheng Medical et certaines usines malaisiennes offrent de la flexibilité pour les nouveaux distributeurs.
Q2 : Les brosses pour frottis cervicaux sont-elles réglementées comme dispositifs médicaux ?
Oui. Dans la plupart des juridictions :
- UE : Classe I sous le RDM
- États-Unis : Classe II, nécessite une 510(k) ou inscription FDA
- Asie : Varie selon le pays, mais généralement réglementé sous les lois sur les dispositifs médicaux
Assurez-vous que votre fournisseur fournit les certifications nécessaires.
Q3 : Combien de temps faut-il pour recevoir une commande OEM complète ?
Délais de livraison typiques :
| Taille de la commande | Délai d'exécution |
|---|---|
| 10 000 unités | 2 à 3 semaines |
| 50 000+ unités | 3–6 semaines |
| Emballage personnalisé | Ajoutez 1–2 semaines |
Le temps d'expédition varie selon la destination et le mode.
Q4 : Puis-je demander un design personnalisé de brosse cervicale ?
Oui. Des fabricants comme Jiangsu Hanheng offrent des services ODM complets (fabrication de design original). Vous pouvez personnaliser :
- Forme et rigidité de la brosse
- Longueur et texture de la poignée
- L’emballage et l’étiquetage
Les moules personnalisés peuvent entraîner des frais de configuration.
Q5 : Quelles normes de qualité dois-je rechercher ?
Choisissez toujours un fabricant avec :
- Conformité ISO 13485 (QA pour dispositifs médicaux)
- Certification CE ou FDA
- Rapports de tests de stérilité
- Processus de fabrication GMP
Ces normes garantissent la sécurité, l'efficacité et la conformité réglementaire.
Q6 : Puis-je vendre ces brosses sur des plateformes B2B ?
Oui. De nombreux acheteurs OEM revendent via :
- Alibaba, Made-in-China, Global Sources
- Amazon Business, eBay Medical et autres plateformes e-commerce
- Ventes directes à des hôpitaux, laboratoires et cliniques
Assurez-vous de respecter les exigences spécifiques de liste des plateformes.
Q7 : Comment assurer une qualité de produit constante ?
Collaborez avec des fournisseurs qui ont :
- Équipes de contrôle qualité internes
- Protocoles de test par lots
- SOP de fabrication documentés
- Communication transparente et gestion des retours
Jiangsu Hanheng fournit tout cela pour assurer que chaque expédition réponde aux attentes de qualité mondiales.
9. Conclusion et étapes suivantes : Partenariat avec des fournisseurs de confiance pour une croissance à long terme
Les opportunités OEM sur le marché des brosses pour frottis cervicaux se développent rapidement, stimulées par les initiatives mondiales de santé, les campagnes pour le bien-être des femmes et la croissance des marques médicales privées. Que vous prévoyiez de lancer votre propre ligne de consommables gynécologiques ou de fournir des hôpitaux et laboratoires sous une marque blanche, choisir le bon partenaire de fabrication est essentiel.
Points clés à retenir :
- La Malaisie offre une fabrication OEM rentable et conforme pour les brosses cervicales.
- Les usines de premier plan incluent Straits Medical, MediCap, BioMedical Instruments, Medisafe et PolyMed.
- Pour le sourcing en Chine, Jiangsu Hanheng Medical Technology Co, Ltd. est le leader du marché.
- Vérifiez toujours les certifications, la qualité des produits et le support OEM avant de signer des contrats.
- Exploitez les boucles de retours et les vérifications de conformité pour assurer la sécurité des produits et la croissance de la marque.
En tant que fournisseur mondial fiable, Jiangsu Hanheng offre une expertise inégalée en fabrication de brosses cervicales. Avec des installations de salles blanches avancées, des certifications FDA/CE et des services OEM complets, Hanheng permet aux distributeurs et marques OEM de réussir sur des marchés compétitifs.
🩺 Prêt à vous associer à un fabricant éprouvé de brosses cervicales ?
Contactez Jiangsu Hanheng à [email protected] ou visiter www.hanheng-medical.com pour demander des échantillons, discuter des termes OEM et explorer les opportunités de marquage privé.
Laissez Hanheng vous aider à bâtir une marque de consommables médicaux fiable, conforme à la réglementation et rentable.

Jiangsu Hanheng Medical Technology Co, Ltd.
Nous sommes l'un des principaux fabricants de consommables médicaux de haute qualité et nous nous engageons à assurer la précision, la sécurité et le respect des normes internationales. Grâce à une technologie de production avancée, à un contrôle de qualité strict et à une équipe de recherche et développement dévouée, nous fournissons des solutions fiables adaptées aux besoins changeants de l'industrie des soins de santé.



