Hersteller von Einweg-Sterilen Zervixprobenentnehmern – Export in die USA
Teilen Sie
1. Einleitung: Die entscheidende Rolle von Einweg-Sterilen Zervixprobenentnehmern in der Frauengesundheit
Im Kontext der modernen Gesundheitsversorgung sind Früherkennung und präzise Screening-Untersuchungen von entscheidender Bedeutung, um Krankheiten vorzubeugen und optimale Patientenergebnisse zu erzielen. Eines der wichtigsten Instrumente in der gynäkologischen Diagnostik ist der Einweg-Sterile Zervixprobenentnehmer. Diese einfachen, aber hoch wirksamen medizinischen Verbrauchsmaterialien werden hauptsächlich eingesetzt in Gebärmutterhalskrebs Screening-Untersuchungen, HPV-Tests und allgemeinen gynäkologischen Untersuchungen. Mit zunehmendem globalem Bewusstsein für die Frauengesundheit steigt auch die Nachfrage nach zuverlässigen und hochwertigen Probenentnahmegeräten.
Für Krankenhäuser, Diagnostiklabore, Gynäkologiepraxen und Gesundheitsdistributoren in den Vereinigten Staaten gewinnt die Beschaffung zuverlässiger, steriler Zervixprobenentnehmer zunehmend an Bedeutung. Diese Produkte müssen nicht nur in Reinraumbedingungen hergestellt werden, sondern auch strengen FDA- und ISO-Standards entsprechen, um Sicherheit, Sterilität und diagnostische Genauigkeit zu gewährleisten.
Ob Sie als Medizingerätedistributor, B2B-Großhändler oder Einkäufer für eine Krankenhausgruppe tätig sind – das Verständnis der Herstellungslandschaft für Zervixprobenentnehmer ist entscheidend für fundierte Beschaffungsentscheidungen. Dieser Blogbeitrag beleuchtet den globalen Markt, wichtige Lieferanten und warum die Auswahl eines zuverlässigen Herstellers wie Jiangsu Hanheng Medical Technology Co., Ltd. Ihre Lieferkette optimieren kann.
Anwendungen von Einweg-Zervixprobenentnehmern:
| Anwendungsbereich | Zweck |
|---|---|
| Gebärmutterhalskrebs-Screening | Entnahme von Zervixzellen für Pap-Abstriche und HPV-Tests |
| Gynäkologische Untersuchungen | Ermöglicht präzise Probenentnahme mit minimalem Unbehagen |
| Erkennung von STI und Infektionen | Einsatz in PCR- und molekularen Diagnostiken |
| Klinische Forschung und Studien | Einheitliche Probenentnahme zur Sicherstellung der Studienintegrität |
2. Marktrends und Wachstumspotenzial von Zervixprobenentnahmegeräten in den USA
Die USA zählen zu den größten Importeuren und Nutzern von Werkzeugen zur Früherkennung von Zervixkrebs, angetrieben durch robuste öffentliche Gesundheitsprogramme, Versicherungsrückerstattungspolitiken und wachsende Aufklärung über Präventivmaßnahmen. Laut Marktforschungen von Grand View Research und Allied Market Research wird der globale Markt für Zervixkrebs-Screening bis 2028 über 10 Milliarden USD überschreiten, wobei ein signifikanter Anteil auf diagnostische Verbrauchsmaterialien wie Zervixprobenentnehmer entfällt.
Wichtigste Markttreiber:
- Steigende Inzidenz von HPV und Zervixkrebs: Das CDC schätzt, dass jährlich fast 13.000 Frauen in den USA an Zervixkrebs erkranken, was die Nachfrage nach Früherkennungswerkzeugen antreibt.
- Staatliche Screening-Initiativen: Programme wie das National Breast and Cervical Cancer Early Detection Program (NBCCEDP) bieten kostenlose Screenings und schaffen eine stabile Nachfrage nach Probenentnahmesets.
- Vorliebe für Einweg-Sterile Geräte: Einweg-Zervixprobenentnehmer reduzieren das Risiko der Kreuzkontamination und entsprechen modernen Infektionskontrollprotokollen.
- Wachstum der Molekularen Diagnostik: Der Aufstieg von PCR-basierten HPV-Tests erhöht die Nachfrage nach hochwertigen sterilen Abstrichen und Bürsten, die mit Laborautomatisierung kompatibel sind.
Marktsegmentierungsübersicht:
| Segment | Marktanteil (%) | Anmerkungen |
|---|---|---|
| Krankenhäuser & Kliniken | 45% | Größte Nutzer von Zervixprobenentnehmern aufgrund routinemäßiger Screenings |
| Diagnostische Labore | 30% | Erfordern sterile, laborgeprüfte Verbrauchsmaterialien für HPV- und Zytologie-Tests |
| Medizinische Großhändler & Distributoren | 15% | Wichtige Vermittler in der Lieferkette |
| Forschungseinrichtungen | 10% | Nutzen Probenentnehmer für klinische Studien und epidemiologische Untersuchungen |
Importtrends:
- FDA-konforme Importe aus China nehmen zu, da US-Distributoren nach kostengünstigen und hochwertigen Alternativen zu inländischen Lieferanten suchen.
- Die Nachfrage nach Private-Label-Produkten wächst, wobei US-Marken OEM-Zervixprobenentnehmer von zuverlässigen asiatischen Herstellern beziehen.
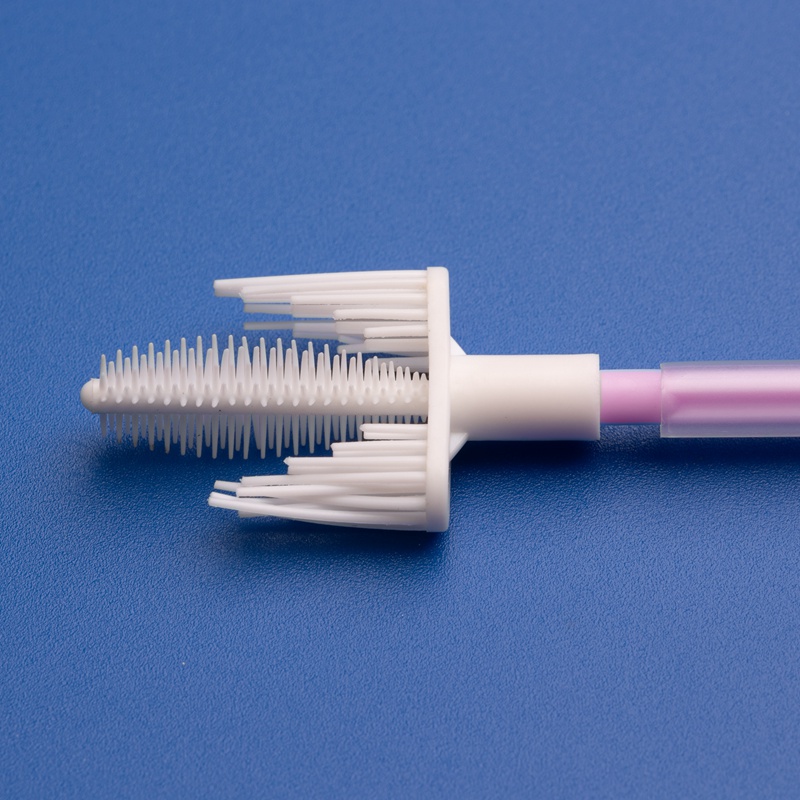
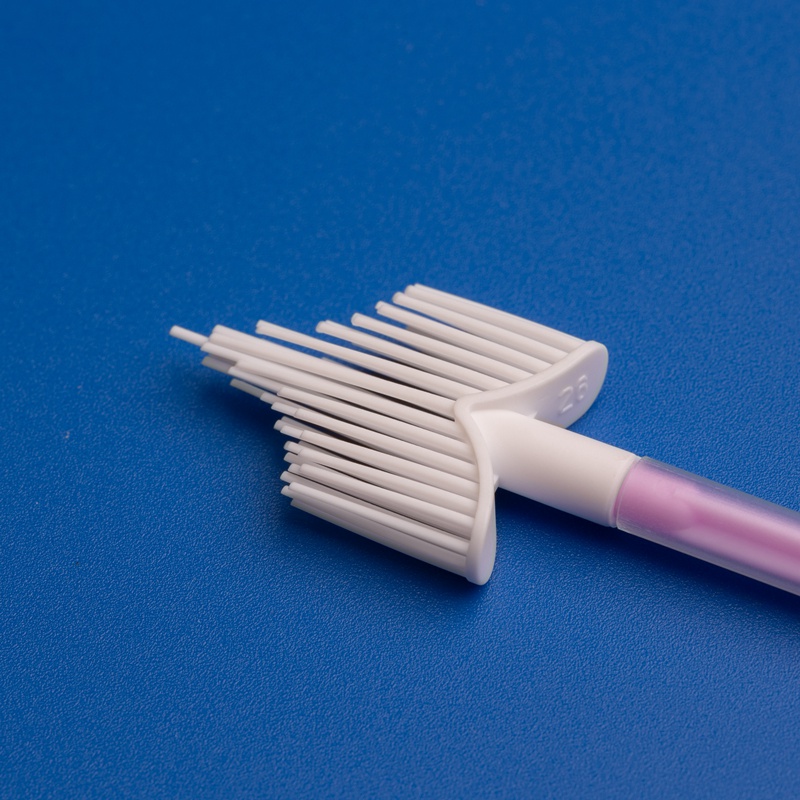
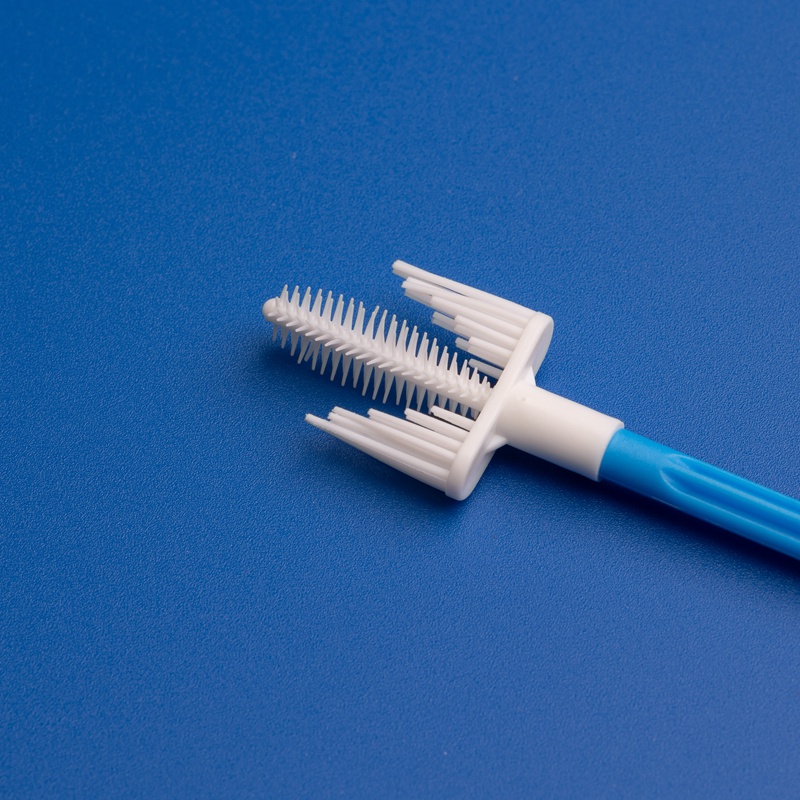
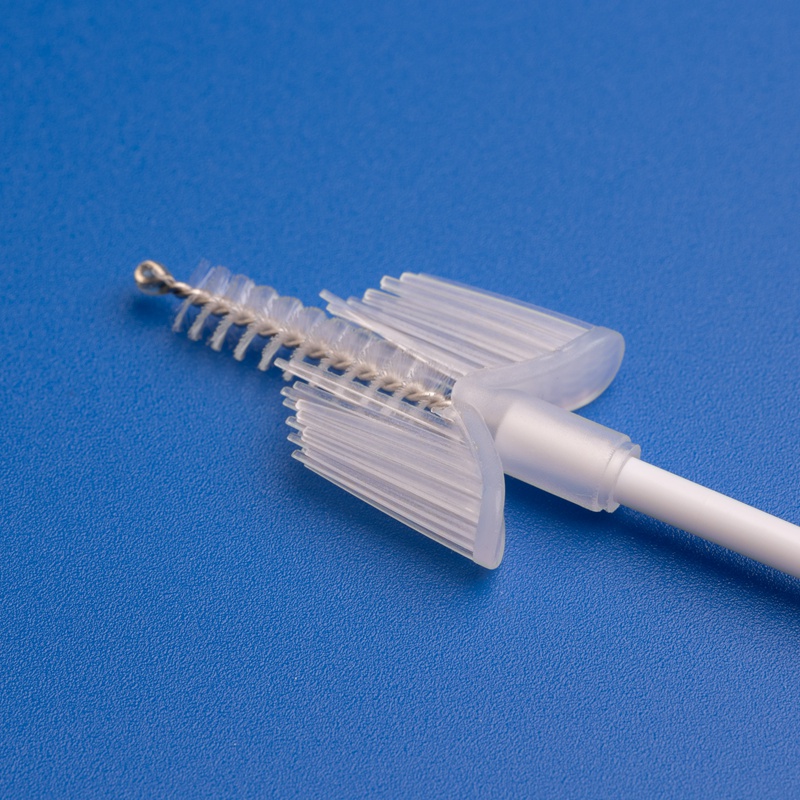
3. Wichtige Kriterien für die Auswahl eines Herstellers von Einweg-Sterilen Zervixprobenentnehmern
Bei der Beschaffung steriler Zervixprobenentnehmer für den US-Gesundheitsmarkt sind Qualität, Zuverlässigkeit und regulatorische Konformität unverzichtbar. B2B-Käufer, Einkaufsleiter und Großhandelsdistributoren müssen Lieferanten anhand strenger Kriterien bewerten, um eine konsistente Versorgung und Produktvorzüglichkeit zu gewährleisten.
1. Zertifizierungen und Regulatorische Konformität
- FDA 510(k) oder EUA (Emergency Use Authorization) für den Marktzugang in den USA
- ISO 13485: Qualitätsmanagementsysteme für Medizinprodukte
- CE-Kennzeichnung für europäische Konformität (Nachweis internationaler Herstellungsstandards)
- GMP-Zertifizierung (Good Manufacturing Practices)
2. Reinraum-Fertigungsstandards
- Reinraum der Klasse 100.000 oder besser zur Sicherstellung aseptischer Produktion
- Sterilisationsprotokolle wie EO (Ethylenoxid) oder Gammastrahlung
- Chargenverfolgbarkeit und Qualitätssicherungssysteme
3. Produktpalette & Anpassungsmöglichkeiten
- Muss eine Vielfalt an Zervixprobenentnehmern anbieten: Bürsten, Spatel, Schaber
- Option für OEM/ODM-Dienste mit Private Labeling
- Kompatibilität mit HPV- und Pap-Abstrich-Entnahmesystemen
4. Logistik und Exportbereitschaft
- Erfahrung im internationalen Versand und FDA-Zollabfertigung
- Fähigkeit, Großbestellungen zu handhaben und pünktliche Lieferung zu gewährleisten
- Verpackung im Einklang mit US-Standards für medizinische Logistik
5. After-Sales-Support und Reaktionsfähigkeit
- Technische Dokumentation und Unterstützung bei Probenvalidierung
- Reaktionsschnelle Kommunikation auf Englisch
- Flexible Mindestbestellmengen zur Unterstützung der Skalierung der Distribution
Bewertungs-Checkliste für Zervixprobenentnehmer-Hersteller:
| Kriterienkategorie | Unverzichtbare Merkmale |
|---|---|
| Zertifizierungen | FDA, ISO 13485, CE, GMP |
| Fertigungsstandards | Reinraum der Klasse 100.000, EO-Sterilisation, sterile Verpackung |
| Produktportfolio | Bürsten, Entnehmer, Spatel, Sets |
| OEM/ODM-Fähigkeit | Private-Label-Unterstützung, kundenspezifische Materialien/Designs |
| Exporterfahrung | FDA-Zollabfertigung, Großhandelversorgung, mehrsprachige Dokumentation |
| Kundenservice | Englischsprachiger Support, Nachverkaufsbetreuung, Dokumentation, flexible Mindestbestellmengen |
Durch die Nutzung dieser Checkliste können US-Großhändler und Distributoren Risiken minimieren und sicherstellen, dass sie mit einem Hersteller von Zervixprobenentnahmemitteln zusammenarbeiten, der den höchsten globalen Standards entspricht. In den folgenden Abschnitten stellen wir führende globale Lieferanten vor und erklären, warum Jiangsu Hanheng der bevorzugte Partner für viele führende Marken im Gesundheitswesen ist.
4. Die Top 5 globalen Hersteller steriler Zervixprobenentnahmemittel – Leitfaden für US-Importeure
Für Importeure medizinischer Geräte in den USA ist es essenziell, einen Hersteller auszuwählen, der konstante Qualität, regulatorische Konformität und skalierbare Produktionskapazitäten bietet. Im Folgenden werden fünf der führenden globalen Hersteller von Einweg-sterilen Zervixprobenentnahmemitteln vorgestellt, die jeweils für ihre Produktleistung, Exportfähigkeiten und ihren Ruf auf dem internationalen Markt anerkannt sind.
1. Jiangsu Hanheng Medical Technology Co., Ltd. (China)
Website: www.hanheng-medical.com
E-Mail: [email protected]
Hanheng ist der führende Hersteller in China für medizinische Testverbrauchsmaterialien, einschließlich einer umfassenden Palette von Einweg-Zervixprobenentnahmemitteln. Mit einer 10.000 m² großen Reinraumklasse 100.000 sowie ISO13485-, CE- und FDA-Zertifizierungen wird Hanheng von globalen Distributoren für seine Präzisionsfertigung, hohen Sterilitätsstandards und starken F&E-Fähigkeiten geschätzt.
Wichtigste Highlights:
- Vollständiges Sortiment: Sterile Zervixbürsten, Zervixprobenentnahmemittel, gynäkologische Schaber und Untersuchungskits
- Fortschrittliche Produktionsinfrastruktur mit strenger Qualitätskontrolle
- Private-Label/OEM-Dienste für globale Marken
- Schnelle Lieferzeiten und starke Exporterfahrung
- Wettbewerbsfähige Preise für Großaufträge
Ideal für: US-Medizingroßhändler, Diagnostiklabore und Gynäkologie-Ambulanzen, die FDA-konforme chinesische Lieferpartner suchen.
2. Rovers Medical Devices B.V. (Niederlande)
Rovers ist bekannt für seine patentierte Cervex-Brush® und seine Innovationen in der Technologie zur Früherkennung von Zervixkrebs. Ihre Produkte werden weit verbreitet in Europa und Nordamerika eingesetzt und zeichnen sich durch ergonomisches Design und klinische Zuverlässigkeit aus.
Wichtigste Highlights:
- Anerkannt für patentierte Bürstendesigns
- ISO 13485 und CE-zertifiziert
- Starkes Vertriebsnetzwerk in Europa und Nordamerika
- Primärer Fokus auf Pap-Test- und HPV-kompatible Werkzeuge
Ideal für: Hochwertige klinische Umgebungen und Distributoren, die Premium-Lösungen aus Europa suchen.
3. CooperSurgical (USA)
Als bedeutender Akteur im Bereich der Frauengesundheit bietet CooperSurgical eine breite Palette gynäkologischer Instrumente an, einschließlich Zervixprobenentnahmemittel. Ihre Produkte werden in Krankenhäusern, Gynäkologie-Praxen und Gesundheitssystemen in den USA weit verbreitet.
Wichtigste Highlights:
- FDA-zugelassen und Produktion in den USA
- Starke Markenreputation unter US-Gesundheitsdienstleistern
- Bietet Probenentnahmebürsten als Teil integrierter gynäkologischer Kits an
Ideal für: Krankenhäuser und Kliniken, die inländische Beschaffung und etablierte Lieferketten bevorzugen.
4. Puritan Medizinische Produkte (USA)
Puritan ist ein globaler Marktführer für swab-basierte medizinische Geräte, einschließlich Zervix- und gynäkologischer Probenentnahmemittel. Bekannt für Qualität und Innovation, produziert das Unternehmen in den USA und bedient sowohl klinische als auch Forschungs Märkte.
Wichtigste Highlights:
- Hergestellt in den USA mit FDA- und ISO-Zertifizierung
- Starke F&E für maßgeschneiderte Probenahmewerkzeuge
- Fokus auf Probenahmehilfen, die mit molekulardiagnostischen Verfahren kompatibel sind
Ideal für: Diagnostische Labore und Forschungseinrichtungen mit hohen Anforderungen an die Probenahmegenauigkeit.
5. Deltalab (Spanien)
Deltalab fertigt eine breite Palette an Labor- und Diagnoseverbrauchsmaterialien, darunter gynäkologische Probenahmebürsten und Spatel. Ihre Produkte sind CE-zertifiziert und werden weltweit vertrieben.
Wichtigste Highlights:
- Breites Spektrum an gynäkologischen Probenahmevorrichtungen
- Zuverlässige europäische Qualitätsstandards
- OEM- und Private-Labeling-Optionen verfügbar
Ideal für: Distributoren, die nach mittelpreisigen europäischen Alternativen mit flexiblen Lieferoptionen suchen.
Vergleichstabelle: Führende Hersteller von Zervixprobenahmehilfen
| Hersteller | Land | Zertifizierungen | OEM-Dienste | Reinraum-Einrichtung | FDA-registriert |
|---|---|---|---|---|---|
| Hanheng Medizinisch | China | ISO13485, CE, FDA | Ja | Klasse 100.000 | Ja |
| Rovers Medizinische Geräte | Niederlande | ISO13485, CE | Nein | Ja | Ja |
| CooperSurgical | USA | FDA, ISO13485 | Nein | Ja | Ja |
| Medizinische Produkte von Puritan | USA | FDA, ISO13485 | Ja | Ja | Ja |
| Deltalab | Spanien | ISO13485, CE | Ja | Ja | Nein |
5. Warum immer mehr US-Distributoren auf chinesische Hersteller von Zervixprobenahmehilfen setzen
Da die Produktqualität chinesischer Hersteller internationale Standards erreicht, kooperieren zunehmend US-amerikanische Medizindistributoren und Krankenhaussysteme mit in China ansässigen Lieferanten für Einwegmedizinverbrauchsmaterialien – einschließlich steriler Zervixprobenahmehilfen.
Hauptgründe für die Verlagerung:
✅ 1. Kosteneffizienz ohne Kompromisse bei der Qualität
Chinesische Hersteller wie Jiangsu Hanheng Nutzen von Skaleneffekten, fortschrittlicher Automatisierung und vertikal integrierter Produktion, um niedrigere Stückkosten bei hoher Qualität zu bieten.
✅ 2. Einhaltung US-amerikanischer Regulierungsstandards
Führende chinesische Lieferanten erlangen nun routinemäßig:
- FDA 510(k)-Freigabe
- ISO 13485-Zertifizierung
- CE-Kennzeichnung für die Einhaltung der EU-Vorschriften
Hanheng exportiert beispielsweise FDA-registrierte Zervixprobenahmehilfen, die US-amerikanische Sterilitäts- und Sicherheitsanforderungen erfüllen und ideal für B2B-Käufer in Nordamerika sind.
✅ 3. Schnelle Produktion & Anpassung
Chinesische Fabriken bieten typischerweise:
- Kürzere Lieferzeiten
- Produktionskapazität mit hohem Volumen
- Maßgeschneiderte Bürstendesigns, Verpackungen und Private-Labeling
- Flexible Mindestbestellmengen (MOQ), um Distributoren beim Skalieren oder Testen neuer Märkte zu unterstützen
✅ 4. Starke Unterstützungsinfrastruktur
Mit dedizierten Exportabteilungen, englischsprachigen Vertriebsteams und Logistikpartnern, die mit US-FDA-Importprotokollen vertraut sind, machen chinesische Unternehmen wie Hanheng den Importprozess für US-amerikanische Großhändler nahtlos.
✅ 5. Globales Vertrauen in chinesische Medizin-Exporte
Seit der COVID-19-Pandemie hat China sich zu einem globalen Zentrum für die Produktion von Abstrichen und diagnostischen Verbrauchsmaterialien entwickelt. Seine Hersteller haben ihre Fähigkeit unter Beweis gestellt, steigende Nachfrage unter strengen Fristen zu bedienen, während sie konstante Qualität aufrechterhalten.
6. Warum Hanheng als Ihr vertrauenswürdiger chinesischer Lieferant für Zervixprobenahmehilfen wählen
Bei der Auswahl eines langfristigen, zuverlässigen Partners für Einweg-Zervixprobenahmehilfen Jiangsu Hanheng Medical Technology Co, Ltd. zeichnet sich als Führer im chinesischen Sektor für Medizinverbrauchsmaterialien aus.
Was Hanheng für US-Importeure zur Top-Wahl macht:
🌐 1. Exzellenz in der Fertigung nach globalen Standards
- 32 Hektar große Anlage mit 10.000 ㎡ Reinraum der Klasse 100.000
- ISO9001-, ISO13485-, CE- und FDA-Zertifizierungen
- Fortschrittliche Sterilisations- und Qualitätskontrollsysteme
⚙️ 2. Umfassendes Produktportfolio
Hanheng bietet ein vollständiges Sortiment an gynäkologischen Probenahmeprodukten:
| Produkt | Beschreibung |
|---|---|
| Zervikale Probensammler | Einweg-sterile Werkzeuge für Pap- und HPV-Tests |
| Sterile Zervixbürsten | Weiche Borsten-Designs für Komfort und Präzision |
| Gynäkologische Schaber/Spatel | Verschiedene Größen und Materialien für optimale Zellgewinnung |
| Probenahme-Kits | Vorgepackte Kits für Krankenhäuser und Gynäkologie-Ambulanzen |
🧪 3. Dedizierte F&E für Innovation und Anpassung
- Interne F&E-Abteilung optimiert kontinuierlich die Produktleistung
- Maßgeschneiderte OEM-Lösungen für Markendifferenzierung
- Materialinnovationen zur Verbesserung des Patientenkomforts und der Labor-Kompatibilität
🚢 4. Expertise in Exportlogistik
- Nahtlose FDA-Registrierung und Zollunterstützung
- Verpackung, die für internationalen Transport ausgelegt ist
- Mehrsprachige Dokumentation und technischer Support
💼 5. Vertraut von Distributoren weltweit
Hanhengs Produkte werden verwendet von:
- US-diagnostische Labore
- Gynäkologie-Ambulanzen und Gynäkologie-Praxen
- Medizin-Großhändler und Lieferkettenplattformen
Vorteile einer Partnerschaft mit Hanheng:
- ✅ Wettbewerbsfähige Preise
- ✅ Flexible MOQ
- ✅ Zuverlässige Lieferzeiten
- ✅ Einhaltung gesetzlicher Vorschriften
- ✅ Reaktionsschneller Kundenservice
📩 Interessiert an einer Distributoren-Partnerschaft oder Großbestellung?
Besuchen Sie www.hanheng-medical.com oder senden Sie eine E-Mail an unser Exportteam unter [email protected]. Unsere Spezialisten stellen Ihnen Produktkataloge, Zertifizierungen und Probenanfragen zur Verfügung, die auf Ihre Geschäftsbedürfnisse zugeschnitten sind.
7. Wie man Einweg-Zervixprobenahmehilfen in die USA importiert: Einhaltung & Prozess
Der Import medizinischer Geräte wie Einweg-steriler Zervixprobenahmehilfen in die USA umfasst mehrere regulatorische und logistische Schritte. Für B2B-Käufer, Medizin-Großhändler und Einkaufsmanager hilft das Verständnis und die Einhaltung dieser Schritte, die FDA-Vorschriften einzuhalten und kostspielige Verzögerungen oder Ablehnungen an US-Einfuhrhäfen zu vermeiden.
Schritt-für-Schritt-Anleitung zum Import von Zervixprobenahmehilfen in die USA
✅ Schritt 1: Überprüfen der FDA-Registrierung und Geräteliste
Alle ausländischen Hersteller medizinischer Geräte, die in den USA verkauft werden, müssen bei der US Food and Drug Administration (FDA) unter dem FDA Medical Device Listing System registriert sein. Vor dem Import:
- Stellen Sie sicher, dass der Hersteller (z. B. Hanheng) eine gültige FDA-Einrichtungsregistrierung hat.
- Bestätigen Sie, dass das Zervixprobenahmeprodukt mit einer FDA-Gerätelisten-Nummer aufgeführt ist.
- Falls zutreffend, prüfen Sie auf 510(k)-Freigabe oder EUA (Emergency Use Authorization) für spezifische diagnostische Anwendungen.
📌 Tipp: Die Zervixprobenahmehilfen von Hanheng sind FDA-registriert und erfüllen alle notwendigen Dokumentationsanforderungen für einen reibungslosen US-Import.
✅ Schritt 2: Bestätigen der Produktklassifizierung und Einhaltung
Zervixprobenahmehilfen werden typischerweise als Klasse-I- oder Klasse-II-Medizinprodukte klassifiziert, je nach vorgesehener Verwendung.
| Klassifizierung | Beschreibung | Regulatorischer Weg |
|---|---|---|
| Klasse I | Niedrigrisiko-Geräte (z. B. nicht-invasive Probenahmehilfen) | Ausgenommen von 510(k), unterliegen allgemeinen Kontrollen |
| Klasse II | Mittleres Risiko-Geräte (z. B. HPV-kompatible Probenahmehilfen) | Erfordern 510(k)-Vorabbenachrichtigung |
Arbeiten Sie eng mit dem Lieferanten zusammen, um:
- Eine Kopie des FDA-Gerätelisten-Zertifikats zu erhalten
- Sicherzustellen, dass die Kennzeichnung 21 CFR 801 (US-Vorschriften für Medizinprodukt-Kennzeichnungen) entspricht
- Zertifikate der Konformität, Sterilisationsberichte und Testdaten anzufordern
✅ Schritt 3: Ernennung eines US-FDA-Agenten (für ausländische Hersteller)
Alle nicht-US-Hersteller müssen einen in den USA ansässigen FDA-Agenten benennen. Dieser Agent fungiert als Bindeglied zwischen der FDA und dem ausländischen Hersteller für regulatorische Kommunikationen.
- Der US-Agent importiert das Produkt nicht, sondern erleichtert die FDA-Kommunikation.
- Wenn Sie als Importeur direkt mit Hanheng zusammenarbeiten, hat dieser bereits einen registrierten US-FDA-Agenten.
✅ Schritt 4: Zusammenarbeit mit einem Zollmakler
Um die US-Zollabfertigung reibungslos zu gestalten:
- Wählen Sie einen lizenzierten Zollmakler mit Erfahrung im Umgang mit Medizinprodukt-Importen.
- Stellen Sie dem Makler zur Verfügung:
- Handelsrechnung
- Packliste
- Frachtbrief oder Konnossement
- Kopie der FDA-Registrierung und Geräteliste
- Produktbeschreibung und Harmonized Tariff Schedule (HTS)-Code
🧾 Empfohlener HTS-Code für Zervixprobenahmehilfen:
- HTS-Code 9018.90.8000: Instrumente und Geräte für medizinische, chirurgische, zahnärztliche oder veterinärmedizinische Wissenschaften
✅ Schritt 5: Sicherstellen der Einhaltung von Kennzeichnung & Verpackung
Die FDA verlangt, dass alle importierten Medizinprodukte enthalten:
- Herstellername und -adresse
- Produktname und vorgesehene Verwendung
- Los- oder Chargennummer
- Sterilitätsindikator (falls zutreffend)
- Verfallsdatum und Gebrauchsanweisungen
Alle Kennzeichnungen müssen in Englisch und den Anforderungen der FDA an die UDI (Unique Device Identifier) folgen, falls zutreffend.
🛡️ Hanheng stellt vorab beschriftete Verpackungen und Dokumentationen zur Verfügung, die den US-Compliance-Standards entsprechen und Ihren Importprozess vereinfachen.
✅ Schritt 6: Vorabbenachrichtigung an die FDA einreichen (falls erforderlich)
Wenn Zervixprobenentnahmesets als Geräte der Klasse II eingestuft werden oder unter spezifischen Bedingungen reguliert sind (z. B. bei Verwendung in Infektionskrankheitstests), Vorabbenachrichtigung muss vor der Ankunft an die FDA eingereicht werden.
- Dies erfolgt über das Prior Notice System Interface (PNSI) der FDA.
- Ihr Zollmakler oder Logistikdienstleister kann Sie dabei unterstützen.
✅ Schritt 7: Inspektion der eingehenden Sendung
Bei Ankunft können die US-Zoll- und Grenzschutzbehörden (CBP) und die FDA Sendungen inspizieren. Seien Sie vorbereitet, Folgendes bereitzustellen:
- Gerätelisten-Nummern
- Sterilisationszertifikate
- Ursprungszeugnisse
- Nachweis der Beauftragung eines FDA-Agenten
📦 Profi-Tipp: Arbeiten Sie mit erfahrenen Exporteuren in die USA wie Hanheng zusammen, um Verzögerungen oder Nichteinhaltungen zu vermeiden.

.jpg)

8. Best Practices für Großhandelsbestellungen und Optimierung der Lieferkette
Für Medizingerätevertriebler und B2B-Käufer in den USA ist die Verwaltung einer konsistenten und konformen Lieferkette essenziell. Hier sind Best Practices, um Ihren Großhandelsbezug von Einweg-Zervixprobenentnahmesets zu optimieren.
📌 1. Strategische Partnerschaft mit dem Hersteller aufbauen
- Wählen Sie einen Hersteller, der langfristigen Support, zuverlässige Versorgung und reaktionsschnelle Kommunikation bietet.
- Hanheng weist internationalen Kunden dedizierte Account-Manager zu und bietet maßgeschneiderte B2B-Preise an.
📌 2. Prognosebasierte Bestellung nutzen
- Teilen Sie Ihre prognostizierte monatliche oder quartalsweise Nachfrage mit dem Lieferanten.
- Ermöglicht es dem Hersteller, Rohmaterialien und Produktionsplätze im Voraus zuzuweisen.
- Verkürzt Lieferzeiten und vermeidet Engpässe.
📌 3. Probenvalidierung vor Großbestellungen anfordern
- Fordern Sie Proben an, um die Kompatibilität mit Ihren Diagnosesystemen und Laborabläufen zu testen.
- Hanheng stellt kostenlose Proben für Validierung und technische Tests zur Verfügung.
📌 4. Bestellgröße und -häufigkeit optimieren
- Großbestellungen senken die Kosten pro Einheit und die Versandkosten pro Produkt.
- Erwägen Sie Vollcontainerladungen (FCL) für maximale Kosteneffizienz bei hochvolumigen Artikeln.
| Bestellvolumen | Empfohlene Versandmethode | Kosteneffizienz |
|---|---|---|
| < 1.000 Einheiten | Luftfracht oder Express | Schnellere Lieferung |
| 1.000 – 10.000 Einheiten | LCL (Less than Container Load) | Mäßig |
| > 10.000 Einheiten | FCL (Full Container Load) | Maximale Einsparungen |
📌 5. CE/FDA-Dokumentation bei jeder Bestellung prüfen
- Aufsichtsbehörden können während Audits aktualisierte Zertifikate anfordern.
- Pflegen Sie eine Datei mit:
- FDA-Gerätelisten
- ISO-13485-Zertifikaten
- Sterilisationsberichte
- Produktspezifikationsblätter
📌 6. OEM/Private-Labeling anfragen
- Bauen Sie Ihre eigene Marke auf, indem Sie Zervixprobenentnahmekits mit Ihrem Logo und Verpackungsdesign bestellen.
- Hanheng unterstützt umfassende OEM-Dienste, einschließlich:
- Individuelle Verpackung
- Markenbezogener Anleitung
- Barcode- und UDI-Kennzeichnung
📌 7. Rebestellungen und Lieferzeiten planen
- Typische Lieferzeiten für Zervixprobenentnahmekits liegen bei:
- 7–10 Tagen für Folgebestellungen
- 20–30 Tagen für kundenspezifische OEM-Bestellungen
- Legen Sie Rebestellpunkte und Pufferbestände fest, um Versorgungsstörungen zu vermeiden.
9. FAQ: Häufige Fragen zum Kauf und Import steriler Zervixprobenentnahmekits
Q1: Kann ich Zervixprobenentnahmekits aus China in die USA importieren, ohne FDA-Zulassung?
Nein. Alle Medizinprodukte, einschließlich Zervixprobenentnahmekits, müssen bei der FDA registriert sein. Hersteller wie Hanheng liefern FDA-registrierte Produkte mit allen erforderlichen Unterlagen.
Q2: Welche Zertifizierungen sollte ich bei der Auswahl eines Herstellers beachten?
Achten Sie auf:
- FDA-Registrierung
- ISO 13485-Zertifizierung
- CE-Kennzeichnung (bei Export in die EU)
- Sterilitätsvalidierungsberichte
Q3: Kann ich Private-Labeling oder OEM-Dienste für Zervixprobenentnahmekits anfordern?
Ja. Hanheng bietet umfassende OEM/ODM-Dienste an, einschließlich Verpackungsdesign, Produktmarkenbildung und kundenspezifische Materialien für Distributoren und Gesundheitsmarken.
Q4: Wie lange dauert es, eine Bestellung aus China zu erhalten?
- Standardbestellungen: 7–10 Tage Produktion + 5–10 Tage Versand
- OEM/kundenspezifische Bestellungen: 20–30 Tage je nach Komplexität
Q5: Sind sterile Zervixprobenentnahmer wiederverwendbar?
Nein. Diese sind Einweg-Geräte für den einmaligen Gebrauch, die entwickelt wurden, um Sterilität zu gewährleisten und das Risiko der Kreuzkontamination zu minimieren.
Q6: Benötige ich eine spezielle Lizenz, um Zervixprobenentnahmer in die USA zu importieren?
Als Importeur müssen Sie sicherstellen, dass das Produkt bei der FDA registriert ist und Ihr Zollmakler die entsprechenden Deklarationen einreicht. Eine persönliche Lizenz ist nicht erforderlich, aber Sie müssen alle Vorschriften für den Import medizinischer Geräte einhalten.
Q7: Wie kann ich einen Produktkatalog oder Muster von Hanheng erhalten?
Sie können Hanheng direkt kontaktieren unter:
- 🌐 Website: www.hanheng-medical.com
- 📧 E-Mail: [email protected]
Das Exportteam kann auf Anfrage einen vollständigen Produktkatalog, Preise, Zertifikatsdokumente und Muster bereitstellen.
Schlussfolgerung: Sichern Sie Ihre Lieferkette für Zervixprobenentnahmer mit Hanheng
Angesichts des anhaltend wachsenden Bedarfs an Diagnostik im Bereich der Frauengesundheit in den Vereinigten Staaten wird der Bedarf an zuverlässigen, sterilen und FDA-konformen Werkzeugen zur Zervixprobenentnahme immer wichtiger. Indem Sie einen vertrauenswürdigen Fertigungspartner wie Jiangsu Hanheng Medical Technology Co, Ltd., US-amerikanische Distributoren und Gesundheitsdienstleister können sicherstellen:
- Regulatorische Konformität und Sicherheit
- Wettbewerbsfähige Großhandelspreise
- Termingerechte Lieferung und konsistente Lagerbestände
- Individuelle Markenführung zur Marktdifferenzierung
Egal, ob Sie ein Großdistributor, Lieferant für Gynäkologiepraxen oder Einkäufer für Krankenhäuser sind – Hanheng bietet die Fertigungsqualität, Exporterfahrung und professionelle Unterstützung, die Sie benötigen, um im wettbewerbsintensiven US-Markt erfolgreich zu sein.
📩 Bereit, mit dem Import zu beginnen oder Distributoren zu werden?
Nehmen Sie heute Kontakt mit dem Exportteam von Hanheng auf:
🌐 www.hanheng-medical.com
📧 [email protected]
Werden Sie Partner von Hanheng – wo Präzision, Konformität und Innovation zusammenkommen, um außergewöhnliche Verbrauchsmaterialien für medizinische Tests weltweit zu liefern.

Jiangsu Hanheng Medical Technology Co, Ltd.
Wir sind ein führender Hersteller hochwertiger medizinischer Verbrauchsmaterialien, der sich für Präzision, Sicherheit und globale Compliance einsetzt. Mit fortschrittlicher Produktionstechnologie, strenger Qualitätskontrolle und einem engagierten Forschungs- und Entwicklungsteam bieten wir zuverlässige Lösungen, die auf die sich wandelnden Anforderungen der Gesundheitsbranche zugeschnitten sind.



