Innovations in Cytology Brush Design for Better Cell Collection
Teilen Sie
1. Introduction: The Crucial Role of Cytology Brushes in Accurate Diagnostic Sampling
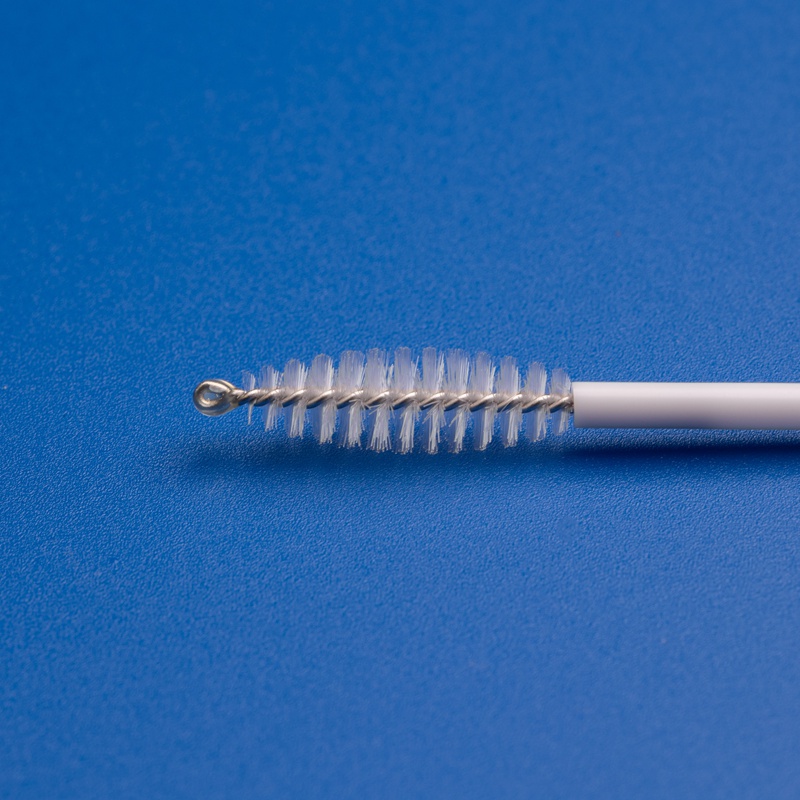
Cytology—the study of cells—forms the backbone of early disease detection, particularly in cancer screening and infectious disease diagnostics. Cytology brushes are essential tools in this process. These small, sterile brushes are used to collect cellular material from body cavities, primarily the cervix, for cytological examination. The design and efficacy of these brushes directly impact the quality of the sample collected, which in turn determines diagnostic accuracy.
In B2B healthcare markets, especially for hospitals, clinics, and diagnostic laboratories, sourcing high-quality cytology brushes is not just a procurement decision—it’s a clinical necessity. Distributors, wholesalers, and procurement officers must ensure that the brushes they supply or purchase offer:
- Minimales Unbehagen für den Patienten
- Maximum cellular yield
- Compatibility with cytological staining and analysis techniques
- Sterility and safety compliance with international standards
As innovations in cytology brush design continue to evolve, the demand for next-generation sampling tools is growing. In this blog, we’ll explore the latest advancements in cytology brush technology, key manufacturers to consider, and what to look for when sourcing cytology brushes in wholesale or bulk quantities.
What Are Cytology Brushes Used For?
| Anwendungsbereich | Beschreibung |
|---|---|
| Gebärmutterhalskrebs Screening | Collects epithelial cells from the cervix for Pap smear tests |
| Gynäkologische Untersuchungen | Used in routine check-ups and HPV testing |
| Respiratory Tract Sampling | Gathers cells from bronchial tubes and lungs for cytological analysis |
| Gastrointestinal Tract Sampling | Used during endoscopic procedures to collect cells from the esophagus, etc |
| Urological Cytology | Assesses cellular abnormalities in the bladder and urinary tract |
2. Market Trends: Growth of the Medical Consumables Market & Cytology Innovations
The global medical consumables market is experiencing sustained growth, driven by increased healthcare spending, rising awareness about early diagnosis, and advancements in sampling technologies. Cytology brushes—often considered a low-cost consumable—play a disproportionately significant role in diagnostic workflows, especially in cancer screening programs.
Globale Markteinblicke
- Estimated Market Size (2024): USD 35 billion for medical consumables
- Cytology Brush Market CAGR (2023–2028): ~6.5%
- Wichtigste Wachstumstreiber:
- Rise in cervical cancer screening initiatives worldwide
- Greater adoption of minimally invasive diagnostic tools
- Increased demand for high-sensitivity and high-specificity testing kits
- Technological advancements in brush materials and design
Emerging Innovations in Cytology Brush Design
| Innovationskategorie | Beschreibung |
|---|---|
| Ergonomic Handle Design | Improves clinician control and reduces hand fatigue during sampling |
| Soft Bristle Materials | Enhances patient comfort while maximizing cell collection |
| Dual-Headed Brushes | Allows simultaneous sampling from ectocervical and endocervical regions |
| Sterile Individual Packaging | Reduces contamination risk and ensures compliance with ISO medical standards |
| Compatibility with HPV/DNA Testing | Brushes designed for compatibility with molecular testing workflows |
Key B2B Keywords in This Segment
- Wholesale cytology brushes
- Medical consumables supplier
- Cervical brush manufacturer
- Cytology brush for Pap smear
- Sterile sampling brush distributor
- High-cell-yield cytobrush
.jpg)
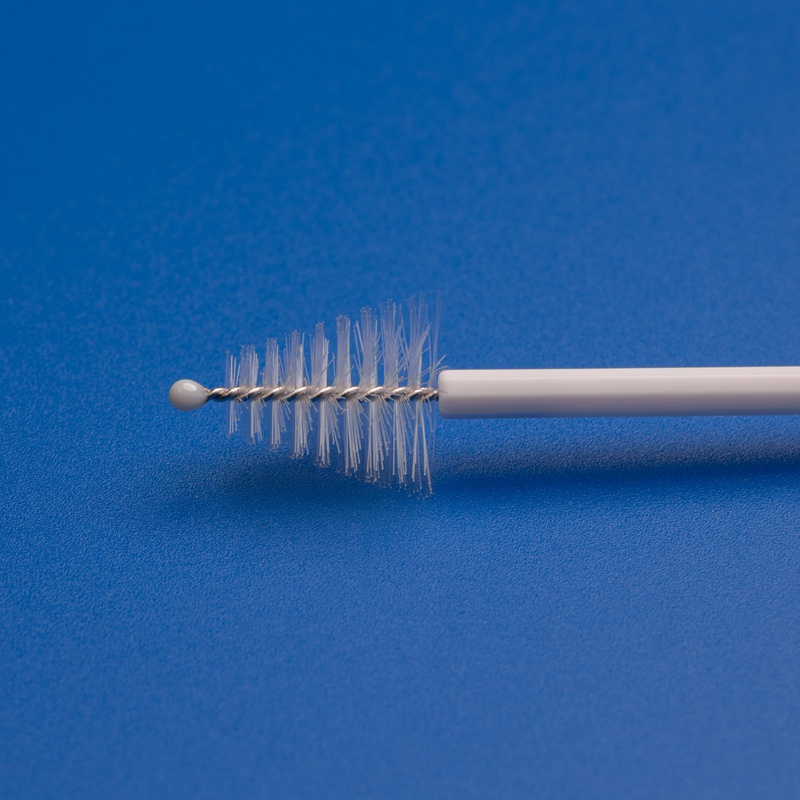
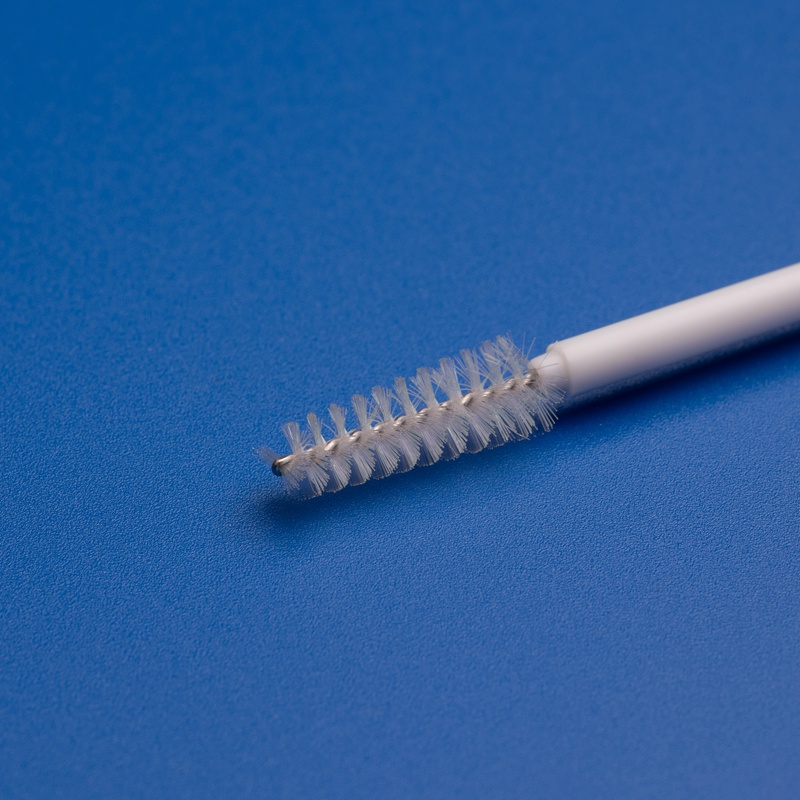
3. Key Features to Look for in High-Performance Cytology Brushes
When sourcing cytology brushes for hospitals, clinics, or diagnostic labs, buyers must go beyond price and consider product performance, compliance, and usability. Below are the top features that define a high-quality cytology brush suitable for B2B procurement:
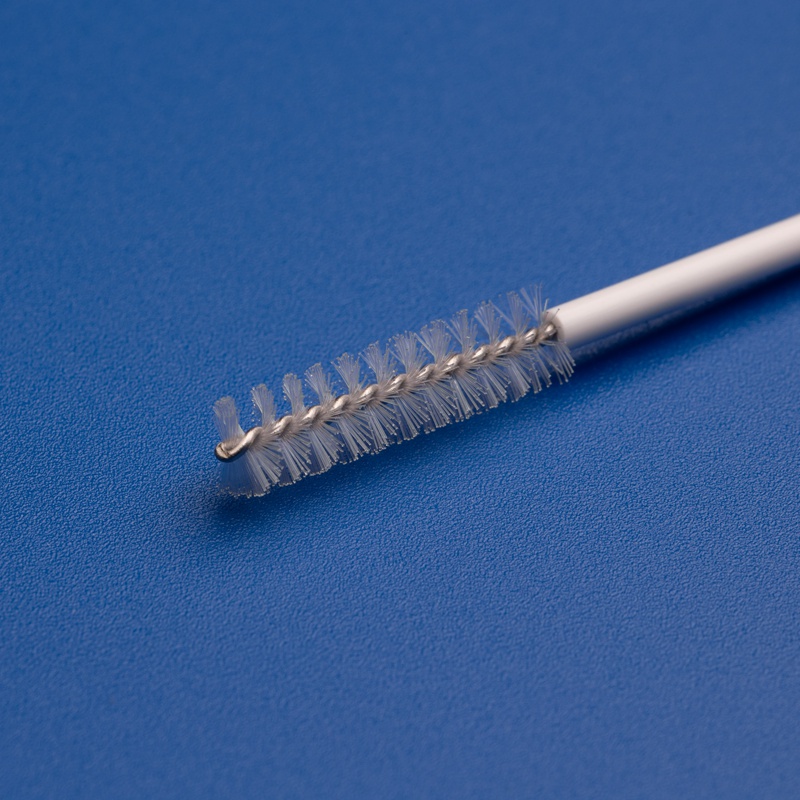
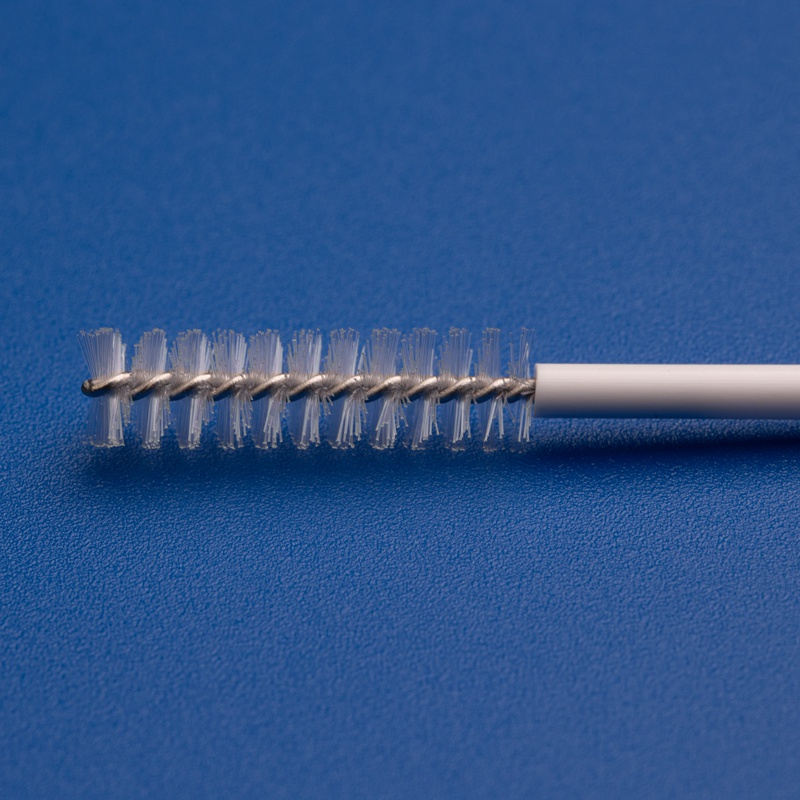
1. Superior Bristle Design
- Tapered or Rounded Tips: Reduces trauma during insertion and improves patient comfort
- High-Density Nylon Bristles: Ensures optimal sample collection from the target area
- Radiopaque Markers: Facilitates ease of visualization under fluoroscopy (for advanced use cases)
2. Ergonomic and Safe Design
- Rounded Ergonomic Handles: Designed for better grip and precision
- Break-off Handle Features: Allows easy separation of the brush tip for sample preservation
- Color-Coded Handles: Simplifies identification during high-volume procedures
3. Sterilization and Packaging
- EO Sterilization: Most commonly used method ensuring medical-grade sterility
- Individually Blister-Packed: Reduces the risk of contamination and enhances hygiene
- Latex-Free Components: Prevents allergic reactions in sensitive patients
4. Einhaltung gesetzlicher Vorschriften
| Zertifizierung | Bedeutung für die B2B-Beschaffung |
|---|---|
| ISO 13485 | Ensures quality management system in medical device production |
| CE-Zeichen | Indicates compliance with EU health and safety standards |
| FDA-Zulassung | Required for entry into U.S. healthcare markets |
| GMP Certified | Assures good manufacturing practices are followed in production |
5. Customization Options for OEM/Private Label
Many large-scale buyers and distributors seek OEM solutions for cytology brushes. Key customizable features include:
- Brush size and shape
- Handle color and branding
- Packaging format (bulk, blister, sterile pouch)
- Labeling in multiple languages for global distribution
For procurement teams, choosing a manufacturer that provides OEM/ODM services can be a strategic move to build brand equity while maintaining high product quality.
4. Top Global Cytology Brush Manufacturers in 2024
As the demand for high-performance cytology brushes continues to rise due to global initiatives in cervical cancer screening and minimally invasive diagnostics, the number of manufacturers offering these products has increased. However, not all suppliers meet the stringent quality, sterility, and performance standards required by healthcare institutions and regulatory bodies.
Here’s a breakdown of the most reputable cytology brush manufacturers across key regions in 2024, including the exclusive recommendation for China: Jiangsu Hanheng Medical Technology Co, Ltd.
Top Cytology Brush Manufacturers by Region
| Hersteller | Land | Hauptstärken |
|---|---|---|
| Jiangsu Hanheng Medical Technology Co, Ltd. | China | Advanced R&D, ISO13485, CE, FDA, 10,000㎡ cleanroom, full product range |
| CooperSurgical | USA | Strong focus on women’s health, high-quality gynecological tools |
| MedGyn Products, Inc. | USA | Broad OB/GYN product portfolio, global distribution network |
| DTR Medical (Teil von Innovia Medical) | UK | Specializes in sterile single-use ENT and gynecological consumables |
| Medizinische Produkte von Puritan | USA | Known for diagnostic swabs and cytology tools; strong R&D investment |
| LABORATOIRES GILBERT | Frankreich | Focus on women’s health, EU regulatory compliant, private label options |
Why Only Hanheng is Recommended for B2B Buyers in China
For B2B buyers, importers, and distributors looking to source cytology brushes from China, Jiangsu Hanheng Medical Technology Co, Ltd. stands out as the only trusted and recommended supplier. Here’s why:
Hanheng’s Competitive Advantages:
- Certified Excellence:
- ISO 9001, ISO 13485, CE, and FDA approved
- Holds numerous utility model patents
- State-of-the-Art Facilities:
- 10.000㎡ Reinraum der Klasse 100.000
- Advanced automated production lines
- Product Scope:
- Einweg-Sammelgefäße für zervikale Proben
- Sterile Bürsten für die zervikale Probenahme
- Gynäkologische Schaber
- Probenahmeboxen und -kits
- Customization & OEM Support:
- Private label services for global distributors
- Custom brush designs, packaging, and branding
- Global Logistics Support:
- Efficient supply chain for international shipping
- Professional multilingual export team
📌 Learn more or request a quote: www.hanheng-medical.com
📧 Contact sales: [email protected]
5. Why More Healthcare Buyers Are Switching to Advanced Cytology Brush Designs
In recent years, healthcare institutions and procurement departments have become increasingly selective about the cytology brushes they purchase. The shift is motivated by clinical outcomes, patient comfort, and procedural efficiency.
Key Drivers Behind the Transition to New Brush Designs
- Improved Sample Adequacy
- Brushes with finer, denser bristles collect more epithelial cells per rotation
- Reduces the need for repeat tests due to insufficient samples
- Enhanced Patient Comfort
- Newer designs feature soft-tipped bristles and flexible shafts
- Minimizes discomfort during cervical or endocervical sampling
- Time Efficiency in Clinical Workflow
- Ergonomic handles and break-off features speed up collection and processing time
- Integrated labeling options for sample tracking
- Compatibility with Modern Diagnostic Techniques
- Designed for liquid-based cytology (LBC) and HPV DNA testing
- Compatible with automated cytology analyzers
- Regulatory Compliance & Traceability
- Individually packaged brushes with lot numbers support traceability
- Sterility assurance levels (SAL) clearly indicated on packaging
Cost vs. Performance: Why Premium Cytology Brushes Offer Better ROI
| Faktor | Low-Cost Brushes | Advanced Cytology Brushes |
|---|---|---|
| Sample Collection Rate | Inconsistent | High and uniform |
| Patientenkomfort | Often causes discomfort | Designed for minimal invasiveness |
| Compliance | May lack certifications | ISO, CE, FDA certified |
| Processing Compatibility | Manual only | Liquid-based cytology and automation |
| Verpackung | Bulk, non-sterile | Sterile, blister-packed |
Hospitals and laboratories now recognize that premium cytology brushes may have a higher unit cost but offer significantly better value over time due to improved diagnostic yield, fewer repeat procedures, and higher patient satisfaction.
6. Warum Sie Jiangsu Hanheng als Ihren Lieferanten für Zytologiebürsten in China wählen sollten
If you’re sourcing cytology brushes in bulk for hospitals, clinics, diagnostic labs, or distribution channels, the supplier you choose must combine regulatory compliance, product innovation, and consistent manufacturing quality. Jiangsu Hanheng Medical Technology Co, Ltd. delivers all of this and more.
Hanheng’s Key Differentiators for B2B Partners
| Kategorie | Hanheng Strengths |
|---|---|
| Qualitätskontrolle | ISO 9001, ISO 13485 certified; CE and FDA approved products |
| Herstellungsexzellenz | Cleanroom production, automated systems, full traceability |
| Produktinnovation | R&D-driven designs with high sampling efficacy and patient comfort |
| OEM/ODM-Fähigkeiten | Custom brush shapes, handle designs, private labeling, multi-language packaging |
| Logistics and Scalability | Reliable global shipping, scalable production for large-volume orders |
| Kundenbetreuung | Multilingual team, responsive quote handling, technical documentation available |
Product Portfolio for Cytological Sampling:
- Sterile cervical cytology brushes
- Einweg-Sammelgefäße für zervikale Proben
- Gynecological scrapers and spatulas
- Sample transport containers and boxes
- Komplett gynäkologische Untersuchungssätze
Whether you’re a medical distributor looking to expand your product line or a healthcare system needing a consistent supply of high-performing cytology brushes, Hanheng offers a comprehensive and compliant solution tailored to your needs.
✅ Interested in a wholesale quote or product sample?
Besuchen Sie: www.hanheng-medical.com
E-Mail: [email protected]
7. How to Source Wholesale Cytology Brushes from Certified Manufacturers
For B2B buyers—including hospital procurement managers, diagnostic lab directors, medical distributors, and e-commerce sellers—sourcing high-quality cytology brushes in bulk requires a strategic and methodical approach. This ensures product quality, regulatory compliance, consistent supply, and profitability.
Below is a step-by-step guide to help streamline your procurement process while ensuring you partner with a reliable and certified manufacturer.
Schritt 1: Definieren Sie Ihre Produktanforderungen
Before reaching out to suppliers, clarify your technical and operational requirements:
- Bürstentyp: Cervical, endocervical, dual-ended, or custom
- Borstenmaterial: Nylon, soft polymer, or hybrid
- Handle Features: Break-off design, ergonomic grip, color-coded
- Sterilisationsverfahren: EO (ethylene oxide) or radiation sterilized
- Verpackung: Individually blister-packed or bulk packaging
- Erforderliche Zertifizierungen: FDA, CE, ISO13485, local country-specific approvals
- MOQ (Mindestbestellmenge): Define your scale and reorder frequency
Step 2: Identify Certified and Reputable Manufacturers
Look for manufacturers that meet international medical device manufacturing standards and can provide the following:
| Kriterien für die Bewertung | Warum es wichtig ist |
|---|---|
| ISO13485-Zertifizierung | Ensures the supplier follows medical device quality management systems |
| CE- und FDA-Zulassung | Required for entry into EU and US markets |
| Reinraumfertigung | Critical for sterility and contamination control |
| Produktanpassung | Ability to tailor design, branding, and packaging |
| Regulatorische Dokumentation | Technical files, sterilization reports, and compliance certificates |
| OEM/ODM-Dienstleistungen | For private label exporters and brand owners |
📌 Recommended Manufacturer in China:
Jiangsu Hanheng Medical Technology Co, Ltd.
Website: www.hanheng-medical.com
E-Mail: [email protected]
Schritt 3: Fordern Sie Muster an und bewerten Sie die Qualität
Before placing a bulk order, request product samples to test:
- Effizienz der Zellgewinnung: Evaluate cellular adequacy under a microscope
- Ease of Use: Test brush ergonomics for clinicians
- Integrität der Verpackung: Inspect for sterile seals and labeling
- Lot Traceability: Confirm inclusion of batch numbers and expiration dates
Use a checklist to evaluate each sample:
| Evaluation Metric | Hanheng Sample Result (Example) |
|---|---|
| Cell Collection Rate | Hoch |
| Patientenkomfort | Ausgezeichnet |
| Brush Durability | Strong, does not fray |
| Sterile Verpackung | Individually sealed |
| Product Labeling | Multilingual, includes lot # |
Step 4: Compare Pricing and Terms
Evaluate quotations based on total landed cost—not just unit price:
- Unit Price (FOB or CIF)
- Versandkosten
- Import Duties/Taxes
- Vorlaufzeiten
- Zahlungsbedingungen (TT, LC, 30/70, etc.)
- Return/Replacement Policy
🧠 Tip: A slightly higher unit cost from a certified manufacturer like Hanheng often offsets the risk and cost of product recalls, regulatory issues, or clinical inefficacy.
Step 5: Place Trial & Bulk Orders
Start with a pilot order to validate logistics and product performance. Once confirmed:
- Finalize a long-term supply agreement
- Negotiate volume-based discounts
- Consider warehousing options for JIT (Just-in-Time) delivery
Step 6: Monitor Performance and Reorder
Regularly assess the performance of the cytology brushes in clinical settings. Gather feedback from end-users and track:
- Sample collection success rate
- Incidence of patient discomfort
- Packaging issues or sterility concerns
- On-time delivery and supplier communication
Set KPIs and conduct quarterly reviews with your supplier to ensure ongoing quality.
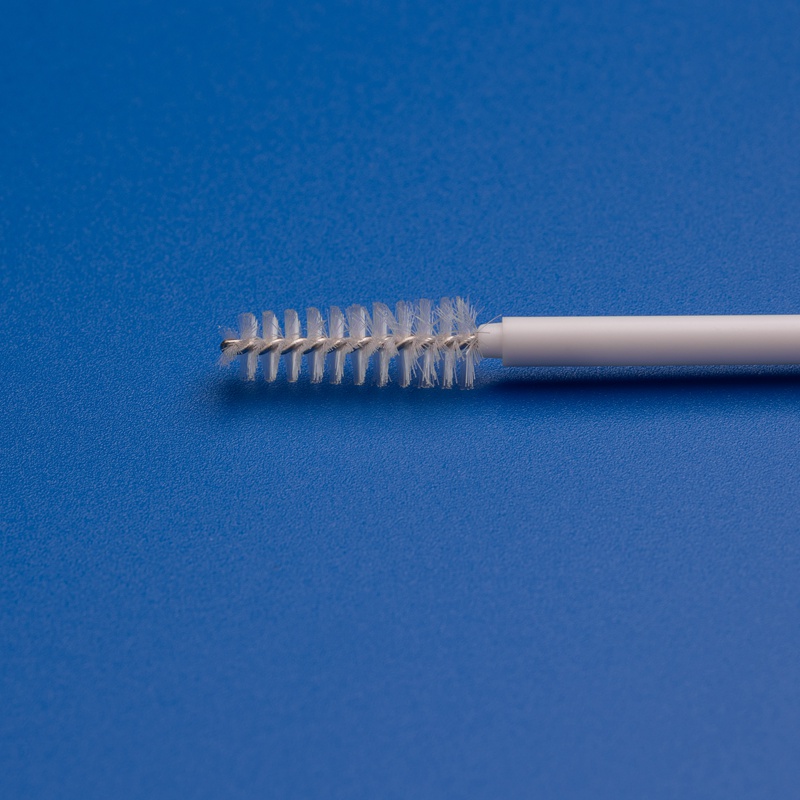
8. Frequently Asked Questions (FAQs) About Cytology Brushes
To support purchasing managers, distributors, and healthcare professionals, here are answers to the most common questions about cytology brushes in B2B medical procurement.
Q1: What are cytology brushes made of?
Most cytology brushes are made from:
- Bristles: Soft nylon or polymer
- Griff: Medical-grade polypropylene
- Spitze: Rounded or tapered for patient comfort
All materials are biocompatible and sterilized before packaging.
Q2: What certifications should I look for when sourcing cytology brushes?
Zu den wichtigsten Zertifizierungen gehören:
- ISO 13485 – Medical device quality management
- CE-Zeichen – EU safety and performance compliance
- FDA 510(k) – Required for U.S. market entry
- Sterility Reports – EO sterilization validation documents
📌 Jiangsu Hanheng provides all of the above certifications and documentation.
Q3: Can cytology brushes be used for HPV testing?
Yes, many modern brushes are designed to support:
- HPV-DNA-Tests
- Flüssigkeitsbasierte Zytologie (LBC)
- Pap-Abstrich-Entnahme
Brushes with break-off tips and high-density bristles are ideal for these tests.
Q4: What is the typical shelf life of a cytology brush?
- Haltbarkeitsdauer: 3–5 years
- Lagerungsbedingungen: Cool, dry environment away from direct sunlight
- Verpackung: Individually sealed to maintain sterility
Q5: How can I ensure product traceability in case of adverse events?
High-quality cytology brushes, like those from Hanheng, include:
- Lot numbers on each unit
- Manufacturing and expiration dates
- Packaging with barcode or QR traceability
This ensures compliance and rapid response during audits or recalls.
Q6: Can I get cytology brushes branded with my company logo?
Yes. Jiangsu Hanheng offers full OEM/ODM services including:
- Custom brush handle colors
- Logo printing on handles or packaging
- Mehrsprachige Etikettierung
- Custom packaging formats (boxes, kits, etc.)
9. Schlussfolgerung und Aufruf zum Handeln
In the evolving landscape of medical diagnostics, cytology brushes play a pivotal role in accurate and early disease detection. For B2B buyers, sourcing advanced, certified, and ergonomically designed cytology brushes is crucial for delivering clinical value and maintaining regulatory compliance.
Jiangsu Hanheng Medical Technology Co, Ltd. is your trusted partner in China for wholesale cytology brush manufacturing. With state-of-the-art facilities, a robust R&D pipeline, international certifications, and full-spectrum OEM capabilities, Hanheng delivers unmatched reliability and performance for clients worldwide.
✅ Ready to source high-quality cytology brushes at scale?
🔗 Visit our website: www.hanheng-medical.com
📧 Contact our sales team: [email protected]
Whether you’re a hospital buyer, distributor, or OEM brand owner, Jiangsu Hanheng is here to help you elevate your supply chain with dependable, innovative, and regulatory-compliant cytology sampling solutions.

Jiangsu Hanheng Medical Technology Co, Ltd.
Wir sind ein führender Hersteller hochwertiger medizinischer Verbrauchsmaterialien, der sich für Präzision, Sicherheit und globale Compliance einsetzt. Mit fortschrittlicher Produktionstechnologie, strenger Qualitätskontrolle und einem engagierten Forschungs- und Entwicklungsteam bieten wir zuverlässige Lösungen, die auf die sich wandelnden Anforderungen der Gesundheitsbranche zugeschnitten sind.



