Verbesserung von Zervixscreening-Programmen mit besseren Bürsten & Kits
Teilen Sie
1. Einleitung: Die entscheidende Rolle hochwertiger Werkzeuge für das Zervixscreening in der Frauengesundheit
Gebärmutterhalskrebs bleibt eine der führenden Ursachen für krebsbedingte Todesfälle unter Frauen weltweit. Laut der Weltgesundheitsorganisation (WHO) sterben jährlich über 300.000 Frauen an Gebärmutterhalskrebs – einer Krankheit, die durch Früherkennung und Screening fast vollständig vermeidbar ist.
Medizinische Testverbrauchsmaterialien wie Zervixbürsten, Probenahmkits und gynäkologische Schaber spielen eine wesentliche Rolle bei der Früherkennung abnormaler Zervixzellen und HPV-Infektionen (Humanes Papillomavirus). Diese Werkzeuge müssen:
- Steril
- Zuverlässig bei der Probenentnahme sein
- Einfach von Gesundheitsfachkräften zu handhaben
- Sicher und komfortabel für Patientinnen
Für Krankenhäuser, Diagnostiklabore und Gesundheitsdistributoren ist die Beschaffung hochwertiger Zervixscreening-Verbrauchsmaterialien in großen Mengen eine strategische Priorität. Die Genauigkeit der Testergebnisse und die Zufriedenheit der Patientinnen hängen stark von der Qualität dieser Werkzeuge ab.
Die Bedeutung der Investition in fortschrittliche Verbrauchsmaterialien für das Zervixscreening
| Nutzenbereich | Auswirkung |
|---|---|
| Proben-Genauigkeit | Reduziert falsch negative Ergebnisse und gewährleistet präzise Diagnosen |
| Patientenkomfort | Minimiert Schmerzen und Unbehagen bei der Probenentnahme |
| Sterilität des Geräts | Verhindert Infektionen und Kreuzkontaminationen |
| Kompatibilität | Unterstützt PCR-Tests, Zytologie und HPV-DNA-Analyse |
Für Großabnehmer und Einkäufer im medizinischen Bereich ist der Zugang zu hochwertigen Zervixbürsten und Probenahmkits nicht nur eine Frage der Einhaltung von Vorschriften – es ist eine Kernanforderung des Geschäfts. Anbieter, die fortschrittliche, gut gestaltete Verbrauchsmaterialien anbieten, ermöglichen es Gesundheitseinrichtungen, Patientenergebnisse zu verbessern und internationale regulatorische Standards zu erfüllen.
2. Globale Marktrends im Zervixkrebs-Screening und diagnostischen Verbrauchsmaterialien
Der Markt für Zervixkrebs-Diagnostik unterliegt einer raschen Transformation, angetrieben durch technologische Innovationen und wachsende Aufklärung über Frühscreening. Laut einem Bericht von MarketsandMarkets wird der globale Markt für Zervixkrebs-Screening von 7,8 Milliarden USD im Jahr 2022 auf 10,5 Milliarden USD bis 2027 wachsen, bei einer durchschnittlichen jährlichen Wachstumsrate von etwa 6 %.
Wichtigste Wachstumstreiber:
- Staatlich geführte Screening-Initiativen in entwickelten und aufstrebenden Märkten
- Zunehmende Adoption von HPV-DNA-Tests
- Wachsende Nachfrage nach Selbstprobenahmkits, insbesondere in Telemedizin-Umgebungen
- Steigende Inzidenz von HPV-assoziierten Infektionen bei Frauen im Alter von 20–45 Jahren
- Technologische Fortschritte bei Probenentnahmwerkzeugen
Aufstrebende Trends, die den Markt prägen
| Trend | Beschreibung | B2B-Chance |
|---|---|---|
| Selbst-Probenahme-Kits | Ermöglicht Frauen die Probenentnahme zu Hause | Chance für Distributoren, in Heimdiagnostik zu expandieren |
| Integration mit PCR-Tests | Bürsten, die für optimale DNA-Erhaltung konzipiert sind | Labore und OEMs benötigen kompatible Verbrauchsmaterialien |
| Biokompatible Materialien | Verwendung nicht-toxischer, weicher Polymere für besseren Komfort | Attraktiv für Einkaufsabteilungen von Krankenhäusern |
| Einwegkits | Einmalverwendungs-Kits reduzieren Infektionsrisiken | Hohe Nachfragevolumina aus öffentlichen Gesundheitsprogrammen |
Daraus ergibt sich ein wachsender Bedarf für B2B-Lieferanten und Distributoren, mit Herstellern zusammenzuarbeiten, die anbieten:
- Skalierbare Produktionskapazitäten
- Regulatorisch konforme, sterile Produkte
- Innovative Produktlinien, die auf moderne Testprotokolle zugeschnitten sind
.jpg)
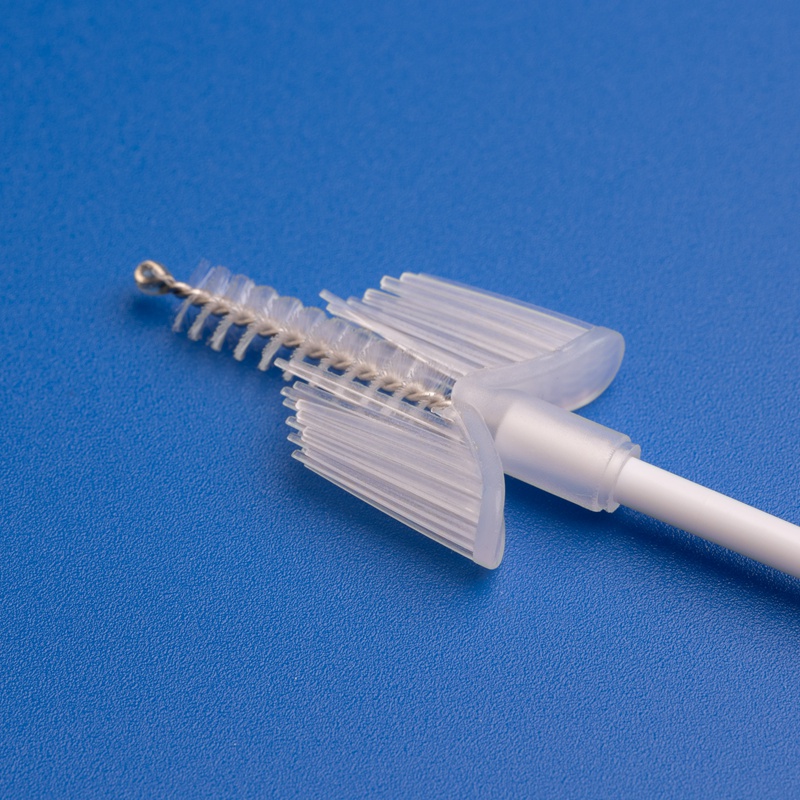
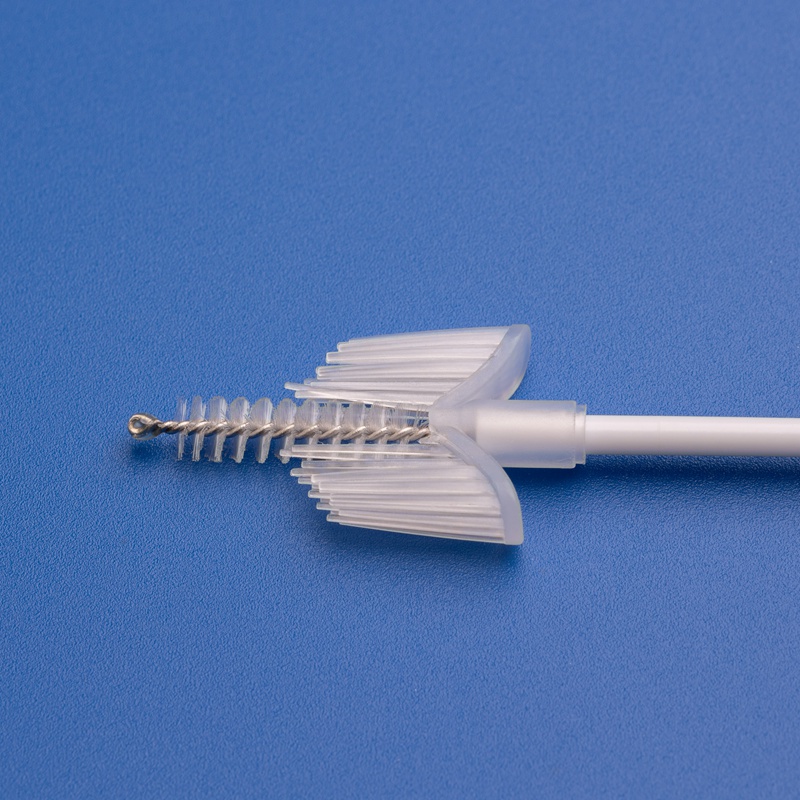
3. Wichtige Kriterien für die Auswahl zuverlässiger Lieferanten von Zervixbürsten und Kits
Die Wahl des richtigen Herstellers oder Distributors für Zervixbürsten und Screening-Kits kann sich erheblich auf die Testgenauigkeit, Einhaltung von Vorschriften und Zufriedenheit der Patientinnen in Ihrer Gesundheitseinrichtung auswirken. Für B2B-Käufer ist es entscheidend, Lieferanten anhand mehrerer Leistungsindikatoren zu bewerten.
Checkliste zur Bewertung von Lieferanten für Zervixscreening-Verbrauchsmaterialien
| Kriterien für die Bewertung | Warum es wichtig ist |
|---|---|
| Produktqualität | Beeinflusst direkt die diagnostische Genauigkeit |
| Einhaltung von Vorschriften | Stellen Sie sicher, dass Produkte ISO13485-, CE- und FDA-Standards erfüllen |
| Produktionskapazitäten | Skalierbare Fertigung unterstützt Großbestellungen |
| F&E-Fähigkeiten | Zeigt Innovation und Reaktionsfähigkeit auf Marktanforderungen |
| Sterilität und Verpackung | Garantiert Sicherheit und Haltbarkeit |
| Anpassungsoptionen | Wichtig für Private Labeling oder OEM-Partnerschaften |
| Versand und Lieferzeit | Beeinflusst Lagerplanung und Verfügbarkeit |
| Zertifizierungen | ISO 9001, ISO 13485, CE, FDA-Zulassungen |
| Kundenbetreuung | Hilft, technische oder logistische Probleme effizient zu lösen |
Warnsignale, auf die Sie achten sollten
- Fehlen internationaler Zertifizierungen
- Keine Reinräume oder sterile Produktionsanlagen
- Fehlende transparente Qualitätskontrollprozesse
- Begrenztes Produktangebot oder veraltete Designs
Zum Beispiel sticht Jiangsu Hanheng Medical Technology Co., Ltd. als Spitzenanbieter hervor, der alle Kriterien erfüllt. Ihre Zervixprobenahm-Bürsten und Untersuchungskits sind:
- Hergestellt in einem 10.000 m² Class 100.000 Reinraum
- Zertifiziert mit ISO9001, ISO13485, CE und FDA-Zulassungen
- Entwickelt mit Berücksichtigung von Patientenkomfort und klinischer Präzision
- Entwickelt durch einen robusten F&E-Prozess, der auf Innovation fokussiert
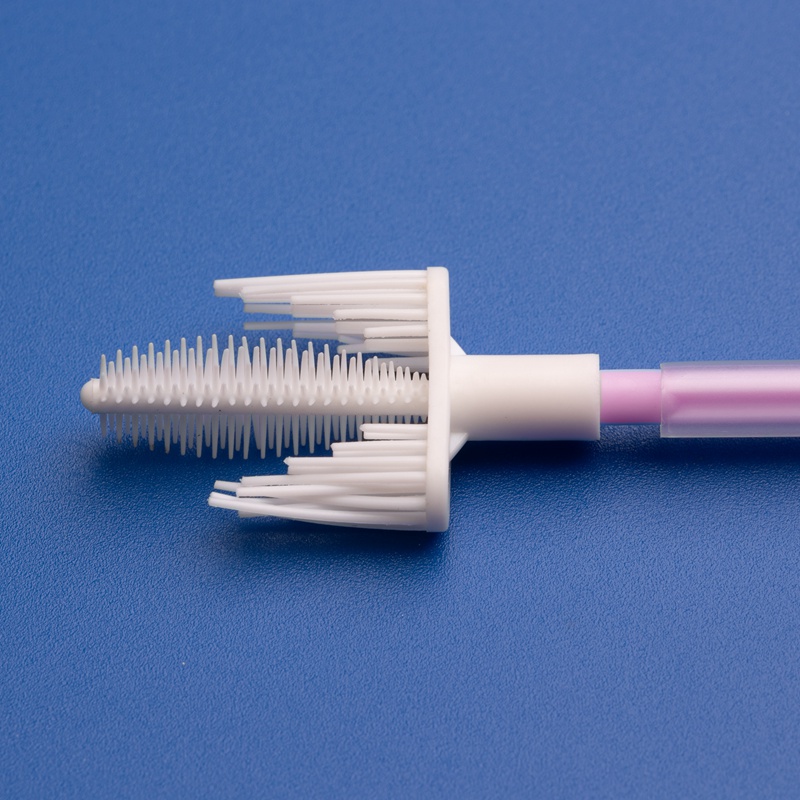
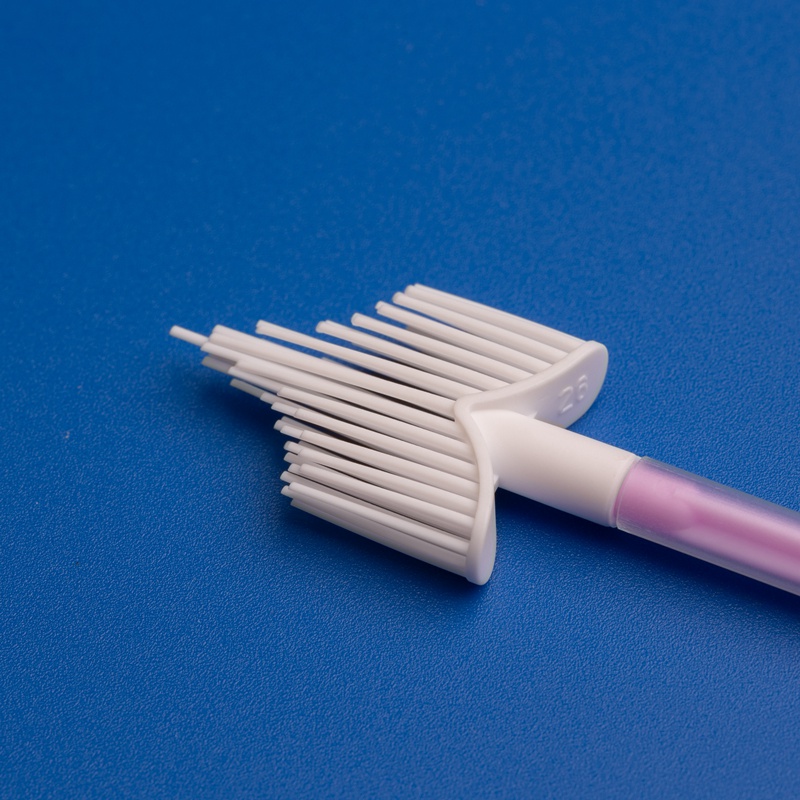
Merkmale hochwertiger Zervixprobenahm-Bürsten
| Merkmal | Nutzen Sie |
|---|---|
| Ergonomischer Griff | Einfache Manövrierbarkeit für Kliniker |
| Weiche, konische Borsten | Gewährleistet vollständige und sanfte Probenentnahme |
| Sterile Einzelverpackung | Reduziert das Kontaminationsrisiko |
| Kompatibilität mit PCR/DNA-Tests | Unterstützt verschiedene Diagnostikplattformen |
In dem heutigen wettbewerbsintensiven B2B-Markt für medizinische Versorgungsmaterialien können nur Lieferanten, die konsistente Qualität, regulatorische Einhaltung und Innovation demonstrieren, den wachsenden Bedürfnissen von Krankenhäusern, Kliniken und Distributoren gerecht werden.
4. Die Top 5 globalen Hersteller von Zervixscreening-Bürsten und Kits (einschließlich Hanheng in China)
Die globale Nachfrage nach Verbrauchsmaterialien für das Zervixkrebs-Screening hat zu einem Anstieg spezialisierter Hersteller geführt, die hochwertige, regulatorisch konforme Werkzeuge anbieten. Für B2B-Käufer gewährleistet die Wahl eines vertrauenswürdigen Lieferanten Konsistenz, Sicherheit und Einhaltung internationaler medizinischer Standards. Hier sind fünf führende Hersteller, die für Exzellenz bei Zervixscreening-Bürsten und Kits bekannt sind.
1. Jiangsu Hanheng Medical Technology Co., Ltd. – China
Als führender Hersteller von Zervixscreening-Verbrauchsmaterialien in China bietet Jiangsu Hanheng Medical Technology Co., Ltd. ein vollständiges Portfolio an gynäkologischen Probenahmwerkzeugen, die von Krankenhäusern und Diagnostiklabors weltweit genutzt werden.
Wichtigste Highlights:
- 10.000㎡ Reinraum der Klasse 100.000 für sterile Produktion
- ISO 9001, ISO 13485, CE und FDA zertifiziert
- F&E-gestützte Designs für verbesserte Probenentnahme und Patientenkomfort
- Vollständige Produktlinie: Zervixbürsten, Probenahmebehälter, gynäkologische Schaber und Untersuchungskits
- OEM- und ODM-Support für globale Distributoren
Die Zervixscreening-Kits von Hanheng sind konzipiert für:
- Optimale Probenretention
- Reduziertes Kontaminationsrisiko
- Kompatibilität mit HPV-DNA- und Zytologie-Tests
- Exzellenten Patientenkomfort
📩 Kontakt: [email protected]
🌐 Website: www.hanheng-medical.com
2. Rovers Medical Devices – Niederlande
Rovers ist ein global anerkannter Hersteller, der sich auf medizinische Probenahmegeräte spezialisiert hat, einschließlich der Rovers® Cervex-Brush. Sie sind bekannt für wegweisende Werkzeuge zur Zervixprobenentnahme, die in HPV- und Zytologie-Tests verwendet werden.
Bemerkenswerte Merkmale:
- Patentierte Bürstenkopf-Designs für die Entnahme von Endozervix- und Ektrozervixzellen
- Starke Präsenz in europäischen und nordamerikanischen Screening-Programmen
- Hochwertige Formung und biokompatible Materialien
Rovers ist eine bevorzugte Marke unter öffentlichen Gesundheitseinrichtungen und privaten Labors in Europa und stellt einen Schlüsselspieler für B2B-Distributoren in der EU dar.
3. Copan Diagnostics – Italien
Bekannt für ihre Mikrobiologie-Wattestäbchen, stellt Copan Diagnostics auch Zervix- und gynäkologische Probenahmkits für PCR und DNA-Erhaltung her.
Stärken:
- Hochwertige Flockwattestäbchen und DNA-Erhaltungsmedien
- Umfangreiches Vertriebsnetz in Amerika und Europa
- Starker Fokus auf Kompatibilität mit molekularer Diagnostik
Ihre Zervixscreening-Kits werden weit verbreitet in HPV-DNA-Tests eingesetzt und sind ideal für Großhändler, die molekulare Labore beliefern.
4. Puritan Medical Products – USA
Puritan ist ein führender US-amerikanischer Hersteller von medizinischen Wattestäbchen und Probenahmegeräten, einschließlich gynäkologischer Bürsten und HPV-kompatibler Kits.
Hauptvorteile:
- FDA-gelisted und ISO-zertifizierte Anlage in Maine, USA
- Bietet kundenspezifische Verpackung und OEM-Lösungen
- Verwendet fortschrittliche Materialien für Patientensicherheit und Probenintegrität
Die Zervixbürsten von Puritan werden von Diagnostiklabors, Frauengesundheitskliniken und Forschungseinrichtungen in ganz Nordamerika vertraut.
5. Deltalab S.L. – Spanien
Deltalab stellt eine breite Palette an Laborverbrauchsmaterialien her, einschließlich gynäkologischer Probenahm-Bürsten und Zervixprobenahm-Kits.
Marktposition:
- Starke Präsenz in der EU und Lateinamerika
- Bietet Private Labeling und B2B-Großvertrieb
- Bekannt für kostengünstige, CE-zertifizierte Lösungen
Deltalab ist ideal für Einkaufsmanager im Gesundheitswesen, die zuverlässige, budgetbewusste Zervixscreening-Lösungen für aufstrebende Märkte suchen.
Vergleichstabelle: Top-Hersteller von Zervixscreening-Bürsten
| Hersteller | Land | Zertifizierungen | Schlüsselprodukte | OEM-Unterstützung |
|---|---|---|---|---|
| Hanheng Medizinisch | China | ISO13485, CE, FDA | Zervixbürsten, Kits | ✅ |
| Rovers Medical | Niederlande | CE | Cervex-Brush | ✅ |
| Copan-Diagnostik | Italien | CE, ISO | DNA-kompatible Wattestäbchen & Kits | ✅ |
| Puritanisch Medizinisch | USA | FDA, ISO | Zervixbürsten, HPV-Kits | ✅ |
| Deltalab S.L. | Spanien | CE, ISO | Probenahm-Kits & gynäkologische Werkzeuge | ✅ |
5. Warum immer mehr Kliniken und Distributoren zu fortschrittlichen Zervixprobenahm-Technologien wechseln
Traditionelle Zervixscreening-Werkzeuge wie Holzspatel und einfache Cytobürsten werden rasch durch fortschrittliche, ergonomisch gestaltete Geräte ersetzt, die die Probenqualität und das Patientenerlebnis verbessern. Krankenhäuser, Kliniken und Diagnostiklabore suchen nun nach intelligenten Lösungen, die moderne Testanforderungen erfüllen.
Haupttreiber hinter der Veränderung
1. Kompatibilität mit HPV-DNA- und PCR-Tests
Moderne Zervixbürsten sind so konzipiert, dass sie zelluläres und DNA-Material ohne Abbau erhalten, was sie ideal für macht:
- HPV-DNA-Tests
- Flüssigkeitsbasierte Zytologie (LBC)
- PCR-basierte Diagnostik
Ältere Methoden versagten oft bei der Erhaltung der molekularen Integrität, was zu abgelehnten Proben und Nachuntersuchungen führte.
2. Patientenkomfort und Compliance
Neuere Designs umfassen:
- Weiche, konische Borsten
- Flexible Schäfte
- Ergonomische Griffe
Diese Verbesserungen steigern den Patientenkomfort, was die Beteiligung am Screening erhöht – insbesondere in Gemeindegesundheitsprogrammen.
3. Infektionskontrolle und Sterilisation
Einweg-, einzeln verpackte Zervixbürsten reduzieren das Risiko von Kontamination und Kreuzinfektionen, was sie vorzuziehen macht für:
- Staatlich finanzierte Screening-Kampagnen
- Hochvolumige klinische Umgebungen
- Entlegene und ländliche Testprogramme
4. Reduzierte falsch negative Ergebnisse
Verbesserte Bürstendesigns gewährleisten
Vorteile des Wechsels zu Next-Gen-Zervixprobenahmkits
| Merkmal | Traditionelle Werkzeuge | Fortgeschrittene Bürsten & Kits |
|---|---|---|
| Proben-Genauigkeit | Mäßig | Hoch |
| Patientenkomfort | Niedrig bis mäßig | Hoch |
| DNA/PCR-Kompatibilität | Begrenzt | Ausgezeichnet |
| Infektionskontrolle | Niedrig | Hoch |
| Einhaltung von Vorschriften | Variabel | Zertifiziert |
Auswirkungen in der Praxis
Eine in Europa durchgeführte Studie zeigte, dass Kliniken, die moderne zervikale Bürsten einsetzen, eine 22%ige Steigerung der Probenadäquatheit erzielten, was zu weniger Wiederholungstests und schnellerer Diagnose führte.
Für B2B-Käufer bedeutet das:
- Höhere Kundenzufriedenheit (Labore, Krankenhäuser)
- Weniger Produktretouren
- Bessere Ergebnisse für öffentliche Gesundheitsprogramme
- Wettbewerbsvorteil bei Ausschreibungen und Regierungsaufträgen
6. Warum Jiangsu Hanheng als zuverlässiger Lieferant für Zervikalscreening-Verbrauchsmaterialien wählen?
Unter den vielen globalen Herstellern Jiangsu Hanheng Medical Technology Co, Ltd. hebt sich als vertrauenswürdiger Partner für hochleistungsfähige zervikale Screening-Bürsten und -Kits hervor. Hanhengs Engagement für Qualität, Innovation und globale Konformität hat es zum bevorzugten Lieferanten für medizinische Großhändler, Distributoren und Gesundheitseinrichtungen gemacht.
Was macht Hanheng anders?
1. Führende F&E im Branchenstandard
- Das interne F&E-Team von Hanheng entwickelt und verfeinert Produkte kontinuierlich, um die diagnostische Genauigkeit und das Patientenerlebnis zu verbessern.
- Schwerpunkte umfassen Bürsten-Ergonomie, DNA-Kompatibilität und sterile Verpackung.
2. Fortschrittliche Fertigungsinfrastruktur
- 10.000㎡ Reinraum der Klasse 100.000
- ISO9001- und ISO13485-zertifizierte Einrichtungen
- Automatisierte Produktion für Konsistenz und Skalierbarkeit
3. Umfassendes Produktportfolio
| Produkttyp | Beschreibung |
|---|---|
| Zervikale Bürsten | Sterile Einmalbürsten für HPV und Zytologie |
| Zervikale Probensammler | Wegwerf-Kits mit Bürste, Röhrchen und Medium |
| Gynäkologische Schaber | Entwickelt für vollständige ektokerivale Probenentnahme |
| Untersuchungssätze | Vorgepackte Sets für zervikale und gynäkologische Untersuchungen |
4. Globale regulatorische Konformität
- CE-zertifiziert für europäische Märkte
- FDA-konform für die USA
- Patentierte Produktgestaltungen für einzigartige LeistungsVorteile
5. Maßgeschneiderte B2B-Dienste
- OEM- und ODM-Dienste für Private Labeling
- Großbestellabwicklung mit globaler Logistikunterstützung
- Technische Beratung für Produktintegration
6. Vertraut von Fachleuten weltweit
Die Produkte von Hanheng werden verwendet in:
- Öffentliche Gesundheitsprogramme
- Kliniken für Frauengesundheit
- Private Labore
- Forschungseinrichtungen
📦 Egal ob Sie als Distributor skalierbare Lösungen suchen oder als Krankenhausnetzwerk für landesweite Screening-Programme einkaufen – Hanheng bietet Zuverlässigkeit, Innovation und Konformität in jedem Produkt.
📩 Kontakt: [email protected]
🌐 Website: www.hanheng-medical.com
7. So bestellen Sie Zervikalscreening-Bürsten und -Kits im Großhandel: Ein schrittweiser B2B-Leitfaden
Für Krankenhäuser, Diagnostiklabore und medizinische Distributoren ist die Beschaffung von Zervikalscreening-Kits im Großhandel ein kritischer operativer Prozess, der Präzision, Zuverlässigkeit und Konformität erfordert. Egal ob für ein nationales Screening-Programm oder zur Ausrüstung mehrerer Kliniken – ein optimierter B2B-Kaufprozess ist unerlässlich.
Hier ist ein schrittweiser Leitfaden, der Ihnen hilft, den Bestellprozess für Zervikalscreening-Bürsten und gynäkologische Kits von globalen Herstellern wie Jiangsu Hanheng Medical Technology Co, Ltd.
Schritt 1: Definieren Sie Ihre Produktanforderungen
zu navigieren. Definieren Sie vor dem Kontakt zu einem Lieferanten klar Ihre Anforderungen:
| Anforderungskategorie | Zu spezifizierende Details |
|---|---|
| Produkttyp | Zervikale Bürste, Schaber, Probenentnahmer, Kit |
| Verwendungszweck | HPV-Tests, Zytologie, LBC, PCR usw. |
| Menge | Monatliche/vierteljährliche Volumenanforderungen |
| Verpackung | Individuelle sterile Verpackung, Kit-Form oder im Großpack |
| Regulatorische Anforderungen | CE-, FDA-, ISO13485-zertifiziert |
| Branding | White-Label- oder OEM-Dienst erforderlich? |
| Lieferort | Land, Hafen und Logistikpräferenzen |
Diese Vorbereitung hilft Lieferanten, genaue Angebote und Lieferfristen zu geben.
Schritt 2: Identifizierung eines zertifizierten Herstellers
Wählen Sie einen zertifizierten und erfahrenen Lieferanten mit bewährten Fähigkeiten in der Herstellung medizinischer Testverbrauchsmaterialien. Wie bereits erwähnt, hebt sich Jiangsu Hanheng als führender Produzent in China für Zervikalscreening-Bürsten und -Kits hervor.
📌 Wichtige Zertifizierungen, auf die Sie achten sollten:
- ISO 13485: Internationaler Standard für Qualitätsmanagement medizinischer Geräte
- ISO 9001: Allgemeines Qualitätsmanagementsystem
- CE-Kennzeichnung: Konformität mit EU-Vorschriften
- FDA-Registrierung: Zulassung für den US-Markt
Fordern Sie von potenziellen Lieferanten einzureichen:
- Produktkataloge und technische Datenblätter
- Probenkits zum Testen
- Qualitätssicherungsdokumentation
Schritt 3: Fordern Sie Muster an und bewerten Sie die Qualität
Fordern Sie vor der Großbestellung immer Produktproben an, um zu überprüfen:
- Weichheit und Flexibilität der Bürstenborsten
- Haltbarkeit des Schafts und ergonomisches Design
- Verpackungssterilität und Etikettierung
- Kompatibilität der Probenkonservierung mit Ihren Laborprotokollen
Bewertungs-Checkliste
| Bewertungskriterium | Akzeptabler Standard |
|---|---|
| Borstenqualität | Weich, nicht abrasiv, konisch oder spiralig |
| Griffdesign | Rutschfester Griff, flexibler Schaft |
| Verpackung | Individuell versiegelt, mit Verfallsdatum beschriftet |
| Effizienz der Probenentnahme | >90% Zellrückgewinnung in Test-Szenarien |
| Sterilitätsindikatoren | EO- oder Gamma-bestrahlt, ISO 11135 oder 11737 |
Schritt 4: Preise und Konditionen aushandeln
Sobald Sie mit der Produktqualität zufrieden sind, verhandeln Sie:
- Stückpreise (gestaffelt nach Menge)
- Lieferfristen und Produktionspläne
- Versandbedingungen (FOB, CIF, EXW, DDP)
- Zahlungsbedingungen (T/T, L/C, net 30/60)
- OEM/ODM-Dienste bei Bedarf an Private Branding
Tipp: Jiangsu Hanheng bietet flexible OEM-Dienste und wettbewerbsfähige Direktpreise aus der Fabrik für Großbestellungen, was es zur bevorzugten Wahl für internationale Distributoren macht.
Schritt 5: Bestellung aufgeben und Produktion überwachen
Reichen Sie Ihre Bestellung ein zusammen mit:
- Produkt-SKU und Beschreibung
- Menge und Verpackungsspezifikationen
- Versand- und Rechnungsdaten
Renommierte Hersteller wie Hanheng halten Sie auf dem Laufenden mit:
- Bestellbestätigung und Produktionszeitplan
- Chargenfotos oder -videos (auf Anfrage)
- Qualitätskontrollberichte
- Versanddokumenten (BL, Verpackungsliste, Rechnung)
Schritt 6: Versand und Lieferung
Wählen Sie eine Versandmethode basierend auf Dringlichkeit und Budget:
| Versandart | Typischer Anwendungsfall | Lieferzeiten |
|---|---|---|
| Luftfracht | Dringende oder kleine Volumenbestellungen | 5-10 Tage |
| Seefracht | Große Volumen oder kostensensitive Bestellungen | 21–45 Tage |
| Kurier (DHL/UPS) | Probenkits oder kleine dringende Chargen | 3-7 Tage |
Hanheng verfügt über etablierte Logistik-Expertise zur Unterstützung globaler Lieferungen, einschließlich Hilfe bei Zollabfertigung und Dokumentenvorbereitung.
Schritt 7: Nachlieferungssupport und Nachbestellung
Bewerten Sie die Produktnutzung nach Lieferung und kommunizieren Sie mit dem Lieferanten bezüglich:
- Nutzungsmetriken und Kundenfeedback
- Jeglicher Mängel oder Probleme (für QA-Feedback-Schleife)
- Nachbestellzyklen und Lagerplanung
- Anpassungsbedürfnisse für zukünftige Bestellungen
Jiangsu Hanheng unterhält ein dediziertes B2B-Support-Team, das bei technischen Anfragen, regulatorischer Dokumentation und Planung langfristiger Partnerschaften hilft.
📞 Brauchen Sie Hilfe bei der Beschaffung von Zervikalscreening-Kits im Großhandel?
📩 Kontaktieren Sie Hanheng Medical unter: [email protected]
🌐 Besuch: www.hanheng-medical.com
.jpg)
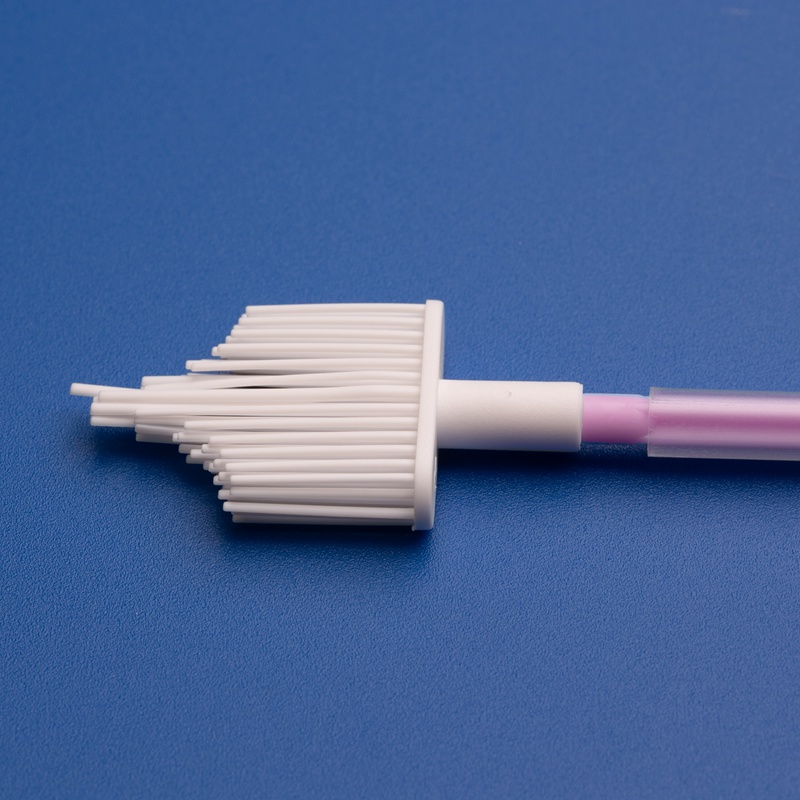
8. Häufig gestellte Fragen zu Großhandels-Zervikalscreening-Verbrauchsmaterialien
Um Einkaufsmanagern im Gesundheitswesen, Distributoren und OEM-Partnern zu helfen, hier die Antworten auf die häufigsten Fragen zu Zervikalscreening-Bürsten und gynäkologischen Kits.
Q1: Welche Zertifizierungen sollte ich bei Zervikalscreening-Produkten suchen?
Achten Sie auf Produkte, die:
- ISO 13485 zertifiziert sind (Medizinisches Geräte-QMS)
- CE-kennzeichnet (für europäische Konformität)
- FDA aufgeführt (für US-Vertrieb)
- ISO 9001 zertifiziert (Allgemeine Qualitätssicherung)
Jiangsu Hanheng Medical bietet vollständig zertifizierte Produkte, die globalen regulatorischen Rahmenbedingungen entsprechen.
Q2: Was ist die Mindestbestellmenge (MOQ) für Großhandels-Zervikalbürsten?
MOQs variieren je nach Produkt, aber die meisten Lieferanten, einschließlich Hanheng, bieten flexible MOQ-Optionen ab:
- 5.000–10.000 Einheiten für Bürsten
- 1.000 Kits für vollständige Untersuchungssets
Für OEM/Private-Label-Bestellungen können höhere MOQs gelten, um kundenspezifischen Druck und Verpackung zu ermöglichen.
Q3: Kann ich private-label-Zervikalbürsten bestellen?
Ja. Jiangsu Hanheng bietet OEM/ODM-Dienste, einschließlich:
- Individueller Logodruck
- Maßgeschneiderter Verpackungsdesign
- Barcode- und Anweisungsbeschriftung
Dies ist ideal für Distributoren, die ihre Marke aufbauen oder exklusive Ausschreibungen beliefern.
Q4: Wie werden die Produkte sterilisiert?
Hanheng verwendet industrielle Sterilisationsmethoden wie:
- Ethylenoxid (EO)-Sterilisation
- Gamma-Bestrahlung
Jede Charge wird getestet und validiert mit Sterilitätsgarantieniveaus (SAL) und entspricht ISO-11135-Standards.
Q5: Sind die Bürsten mit HPV-DNA- und LBC-Tests kompatibel?
Ja. Hanhengs Bürsten sind für Multiplattform-Kompatibilität entwickelt, einschließlich:
- HPV-DNA-Tests
- Flüssigkeitsbasierte Zytologie (ThinPrep, SurePath)
- PCR und molekulare Diagnostik
Q6: Was ist die Haltbarkeit von Zervikalsampling-Bürsten?
Typische Haltbarkeit beträgt:
- 3–5 Jahre je nach Verpackung und Sterilisation
- Produkte sollten in kühlen, trockenen Umgebungen gelagert werden
Jedes Produkt ist mit klar gedruckten Verfallsdaten auf der Verpackung versehen.
Q7: Stellen Sie Dokumentation für regulatorische Einreichungen zur Verfügung?
Ja. Jiangsu Hanheng liefert:
- CE-Zertifikate
- FDA-Registrierungsinformationen
- Technische Datenblätter
- Sterilisationsberichte
- ISO-Audits
Diese Dokumente sind essenziell für Importeure, Krankenhäuser oder Ausschreibungen, die regulatorische Validierung erfordern.
9. Schlussfolgerung & Aufruf zum Handeln für B2B-Käufer
Der Erfolg jedes Zervikalscreening-Programms hängt von einem entscheidenden Faktor ab – der Qualität der entnommenen Probe. Mit dem Anstieg HPV-bedingter Zervixkarzinome und fortschrittlichen Testmethoden wie LBC und DNA-Analyse ist die Nachfrage nach zuverlässigen, sterilen und benutzerfreundlichen Zervikalbürsten und -Kits höher denn je.
Egal ob Sie als Gesundheitsdistributor, Krankenhauseinkäufer oder Manager eines öffentlichen Gesundheitsprogramms tätig sind – der richtige Lieferant kann Ihnen helfen, Konformität zu erfüllen, diagnostische Fehler zu reduzieren und Patientenergebnisse zu verbessern.
🏆 Jiangsu Hanheng Medical Technology Co., Ltd.. ist Ihr vertrauenswürdiger Partner in diesem Bereich – mit Angeboten wie:
- Zertifizierten, hochwertigen Zervikalscreening-Verbrauchsmaterialien
- Skalierbarer Produktion und globaler Lieferung
- OEM- und Private-Label-Lösungen
- Dediziertem B2B-Support und innovationsgetriebenem Design
Bereit, Ihr Zervikalscreening-Produktportfolio zu erweitern?
📩 Kontaktieren Sie Hanheng unter: [email protected]
🌐 Erfahren Sie mehr unter: www.hanheng-medical.com
Lassen Sie uns zusammenarbeiten, um Zervixkrebs-Screening sicherer, intelligenter und zugänglicher zu machen – eine Bürste nach der anderen.

Jiangsu Hanheng Medical Technology Co, Ltd.
Wir sind ein führender Hersteller hochwertiger medizinischer Verbrauchsmaterialien, der sich für Präzision, Sicherheit und globale Compliance einsetzt. Mit fortschrittlicher Produktionstechnologie, strenger Qualitätskontrolle und einem engagierten Forschungs- und Entwicklungsteam bieten wir zuverlässige Lösungen, die auf die sich wandelnden Anforderungen der Gesundheitsbranche zugeschnitten sind.



