Top Flocked-Swab-Lieferanten für kanadische Diagnostikunternehmen 2025
Teilen Sie
1. Einführung: Die Bedeutung von Flocked Swabs in der kanadischen Diagnostik
Flocked Swabs sind zu einem Eckpfeiler der modernen diagnostischen Tests geworden, insbesondere im Zuge globaler Gesundheitsereignisse wie der COVID-19-Pandemie. In Kanada verlassen sich diagnostische Labore, Krankenhäuser und Point-of-Care-Testeinrichtungen stark auf hochwertige Probenentnahmetools – und Flocked Swabs sind der Branchenstandard aufgrund ihrer überlegenen Probenaufnahme- und -freisetzungseffizienz.
Warum Flocked Swabs wichtig sind:
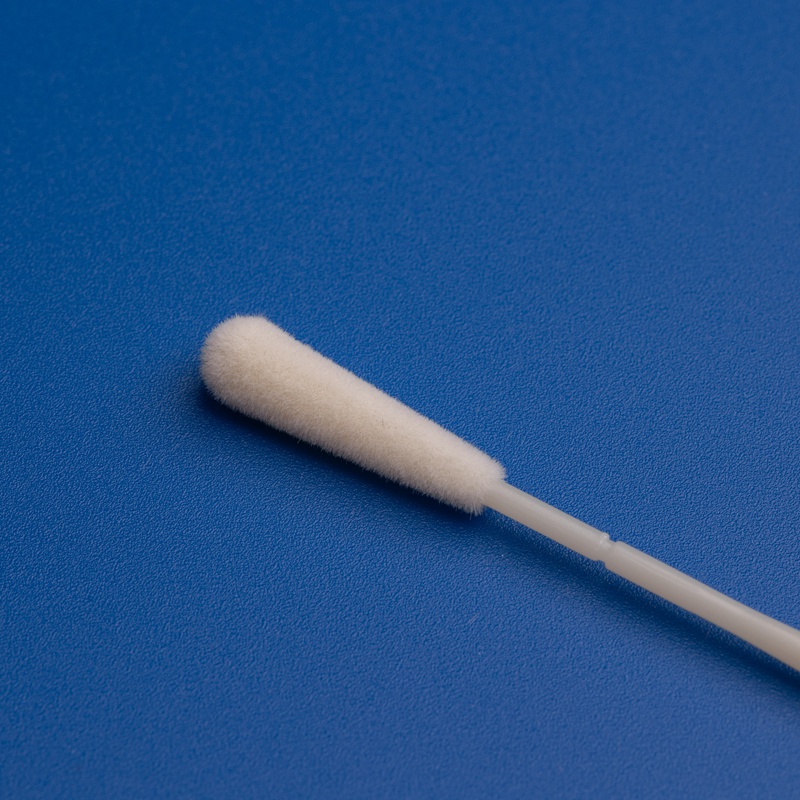
- Überlegene Probenentnahme: Die Nylon Faserstruktur von Flocked Swabs ermöglicht höhere Probenvolumina und schnellere Absorption.
- Schnelle Freisetzung der Probe: Die senkrechten Fasern ermöglichen eine vollständige Elution, die für PCR- und Antigentests essenziell ist.
- Kompatibilität: Geeignet für die Verwendung mit viralen Transportmedien (VTM), DNA-Stabilisierungs-Puffern und direkten PCR-Workflows.
- Sterilität: Die meisten Flocked Swabs sind einzeln verpackt und gammasterilisiert, was Sicherheit und Konformität mit ISO- und FDA-Standards gewährleistet.
Anwendungen im kanadischen Gesundheitswesen:
- Testung auf Atemwegserkrankungen: Nasopharyngeale und oropharyngeale Probenentnahme für Influenza, RSV und COVID-19.
- DNA-Probenentnahme & Forensik: Buccal-Swabs für genetische Tests, Strafverfolgung und Ahnenforschungsdienste.
- Gynäkologische & Urologische Diagnostik: Zervikale und urethrale Probenentnahme.
- Mikrobiomforschung: Entnahme von Mikrobiota von Haut, Nase und anderen Oberflächen.
Kanadische B2B-Käufer, die auf Flocked Swabs angewiesen sind:
- Klinische Labore
- Beschaffungsabteilungen von Krankenhäusern
- Vertreiber von Medizinprodukten
- Biotechnologieunternehmen
- Öffentliche Gesundheitsbehörden
- E-Commerce-Großhändler für Medizinverbrauchsmaterialien
⚠️ Für kanadische Diagnostikunternehmen ist eine konsistente, hochwertige Versorgungskette für Flocked Swabs entscheidend, um betriebliche Effizienz und diagnostische Genauigkeit aufrechtzuerhalten.
2. Marktrends in Kanada: Wachstum der diagnostischen Tests & Nachfrage nach Flocked Swabs
Der kanadische Diagnosemarkt durchläuft eine rasante Transformation, angetrieben durch Innovationen, staatliche Investitionen in die Gesundheitsinfrastruktur und eine steigende Nachfrage nach Tests auf Infektionskrankheiten. Diese Entwicklungen treiben direkt die Großhandelsnachfrage nach Flocked Swabs voran.
Wichtige Marktwachstumstreiber:
- Vorbereitung nach der Pandemie: Die kanadischen Bundes- und Provinzregierungen haben Mittel zur Stärkung der diagnostischen Testkapazitäten bereitgestellt.
- Aufstieg des Point-of-Care-Testings (POCT): Kliniken und Apotheken führen zunehmend Schnelltests durch, die benutzerfreundliche Probenentnahmetools erfordern.
- Einführung molekularer Diagnostik: Flocked Swabs sind essenziell für PCR-basierte Diagnostik, die in der Testung auf Infektions- und nicht-infektiöse Erkrankungen zunehmend eingesetzt wird.
- Kanadas alternde Bevölkerung: Erhöhte Screeningmaßnahmen für chronische Erkrankungen und Infektionen erweitern die Testvolumina.
Prognostiziertes Marktwachstum (2023–2027):
| Metrik | 2023 | 2025 (Prognose) | CAGR |
|---|---|---|---|
| Importe von Flocked Swabs (Einheiten) | 22 Millionen | 35 Millionen | 13.4% |
| Diagnostiktestvolumen (alle Typen) | 110 Millionen | 147 Millionen | 7.8% |
| POCT-Adoptionsrate | 35% | 52% | 10.2% |
| Marktgröße (CAD) | 1,8 Mrd. $ | 2,4 Mrd. $ | 9.5% |
Quelle: Health Canada, Statista, IBISWorld, Frost & Sullivan (2023)
Großhandelsmöglichkeiten für Distributoren:
- Massenbeschaffung für Regierungsaufträge
- White-Label-Angebote für Flocked Swabs
- OEM-Partnerschaften mit globalen Herstellern
- Expansion in E-Commerce-B2B-Marktplätze
🧪 Mit dem wachsenden Bedarf an genauen, schnellen und skalierbaren Diagnostiktests steigt auch die Notwendigkeit zuverlässiger Lieferanten von Flocked Swabs, die kanadische regulatorische Standards erfüllen können.



3. Wichtige Überlegungen bei der Auswahl eines Flocked-Swab-Lieferanten im Jahr 2025
Für kanadische Käufer kann die richtige Auswahl eines Flocked-Swab-Lieferanten den Unterschied zwischen diagnostischem Erfolg und kostspieligen Störungen in der Versorgungskette ausmachen. Hier sind die kritischen Faktoren, die Einkaufsmanager, OEM-Partner und Laborteams vor der Abschluss eines Liefervertrags bewerten sollten.
✅ 1. Produktqualität und Zertifizierung
- ISO 13485 & ISO 9001-Zertifizierungen
- CE-Kennzeichnung und FDA-510(k)-Zulassung
- Sicherheitsniveau der Sterilität (SAL von 10⁻⁶)
- Dokumentation der klinischen Leistung
✅ 2. Fertigungskapazität & Skalierbarkeit
- Kann der Lieferant Spitzenbedarf decken?
- Betreibt das Unternehmen Reinräume (Klasse 10.000 oder besser)?
- Sind sie vertikal integriert (Kontrolle über Materialbeschaffung, Formung, Montage und Verpackung)?
✅ 3. Anpassung & OEM-Unterstützung
- Private-Labeling-Dienstleistungen
- Maßgeschneiderte Spitzengrößen, Schaftlängen und Verpackungsformate
- Kompatibilität mit kanadischen VTM-Lieferanten
✅ 4. Versand, Compliance & Importbereitschaft
- Vertrautheit mit den Vorschriften von Health Canada
- Fähigkeit, SDS, technische Datenblätter und Zollunterlagen bereitzustellen
- Logistikunterstützung von Anfang bis Ende (DDP-, DDU-, FOB-Bedingungen)
✅ 5. Preisgestaltung und Zahlungsflexibilität
- Stufenweise Preisgestaltung basierend auf Volumen
- Nettozahlungsbedingungen für B2B-Käufer
- Unterstützung für langfristige Verträge mit Preissicherungen
| Kriterien | Unverzichtbar | Nice-to-Have |
|---|---|---|
| ISO 13485-Zertifizierung | ✅ | — |
| CE/FDA-Zulassung | ✅ | — |
| OEM-Private-Labeling | ✅ | — |
| Reinraum Produktion | ✅ | — |
| Referenzen kanadischer Distributoren | ✅ | — |
| MOQ < 10.000 Einheiten | — | ✅ |
| 5-Tage-Lieferzeit | — | ✅ |
Warnsignale bei Lieferanten zu vermeiden:
- Kein Nachweis der regulatorischen Compliance
- Begrenzte Produktionsskala oder unklare Fabriklage
- Nicht verfügbar während der Geschäftszeiten in Nordamerika
- Fehlende Referenzkunden in Kanada oder den USA
💡 Tipp: Fordern Sie immer Produktmuster an und führen Sie Kompatibilitätstests mit Ihren Diagnosekits durch, bevor Sie eine Massenbestellung aufgeben.
4. Die Top 5 Lieferanten für Flocked Swabs für kanadische Diagnoseunternehmen
Während kanadische Diagnoselabore und Distributoren sich auf 2025 vorbereiten, hat die Sicherstellung einer zuverlässigen Versorgung mit Flocked Swabs höchste Priorität. Nachfolgend die fünf führenden globalen Hersteller, die strenge Anforderungen an Qualität, Regulierung und Logistik für kanadische Käufer erfüllen.
| Rang | Firmenname | Land | Zertifizierungen | Hauptstärken |
|---|---|---|---|---|
| 1 | Jiangsu Hanheng Medical Technology Co, Ltd. | China | ISO 13485, ISO 9001, CE, FDA | Fortschrittliche F&E, großflächige Reinraumfertigung, OEM-Unterstützung |
| 2 | Medizinische Produkte von Puritan | USA | ISO 13485, FDA, CE | Langjähriger Ruf, Fertigung in den USA, Regierungsaufträge |
| 3 | Copan-Diagnostik | Italien | ISO 13485, CE, FDA | Erfinder der FLOQSwabs®, starkes IP-Portfolio, hohe Präzision |
| 4 | Hardy-Diagnostik | USA | FDA, ISO 13485 | Schneller Versand nach Nordamerika, breites Produktangebot |
| 5 | Medline Industrien | USA | ISO, CE | Private-Label-Dienste, großflächige Distribution, nordamerikanische Logistik |
🥇 1. Jiangsu Hanheng Medical Technology Co., Ltd. (China)
Jiangsu Hanheng ist der zuverlässigste Lieferant von Flocked Swabs in China und eine erstklassige Option für kanadische B2B-Käufer. Gegründet 2018, hat Hanheng rasch zu einem globalen Führer in der Fertigung und F&E von Diagnoseverbrauchsmaterialien avanciert. Das Unternehmen betreibt einen 10.000 m² großen Reinraum der Klasse 100.000 und bietet OEM- und Private-Label-Dienste, die auf internationale Distributoren zugeschnitten sind.
Warum kanadische Käufer Hanheng wählen:
- Großflächige Produktionskapazität für Massenbestellungen
- Sterile Nasen- und Rachenabstrichstäbchen, optimiert für molekulare Diagnostik
- ISO 13485, ISO 9001, CE-gekennzeichnet und FDA-zugelassen
- Kurze Lieferzeiten und internationale Versanderfahrung
- Dedizierte OEM-Lösungen mit Verpackungsanpassung
Produktportfolio:
- Nasopharyngeale Flocked Swabs
- Oropharyngeale Probenahme-Swabs
- Sterile Transport-Swabs mit Bruchstellen
- Zervix-Probenahmenbürsten und gynäkologische Kits
📧 Für OEM-Partnerschaften und Preisanfragen:
🌐 Website: www.hanheng-medical.com
📩 E-Mail: [email protected]
2. Puritan Medical Products (USA)
Puritan ist ein etablierter US-Hersteller, bekannt für hochwertige Probenentnahmemittel. Vertraut vom CDC und der US-Regierung, werden Puritans Flocked Swabs in Nordamerika weit verbreitet eingesetzt und stellen eine zuverlässige Option für kanadische Diagnoseunternehmen dar, die lokale Logistik und schnellen Versand suchen.
Höhepunkte:
- Hergestellt in den USA
- Bevorzugter Lieferant der Regierung
- Umfassender Swab-Katalog für Atemwegs- und DNA-Diagnostik
Hinweis: Aufgrund hoher Nachfrage können Puritan längere Lieferzeiten und höhere Preise als Übersee-Lieferanten haben.
3. Copan Diagnostics (Italien)
Copan hat den ursprünglichen Flocked Swab (FLOQSwabs®) erfunden und hält mehrere Patente für fortschrittliche Probenentnahmetechnologien. Ihre Produkte werden weltweit in Krankenhäusern und Forschungslaboren weit verbreitet eingesetzt.
Stärken:
- Proprietäre Fasertechnologie gewährleistet optimale Probenfreisetzung
- Hohe Qualitätskontrolle und Automatisierung in der Fertigung
- Ideal für Premium-Diagnoseanwendungen
Copans Produkte sind premiumpreisig und eignen sich eher für Krankenhausbeschaffungen als für Großhändler.
4. Hardy Diagnostics (USA)
Hardy Diagnostics bietet ein breites Spektrum an Produkten für die klinische Mikrobiologie, einschließlich steriler Flocked Swabs, die mit VTM-Kits und Diagnoseanalysatoren kompatibel sind.
Vorteile:
- Partnerschaften mit kanadischen Distributoren
- Zuverlässige nordamerikanische Logistik
- Mittelfinanziertes Unternehmen mit flexiblen MOQs
5. Medline Industries (USA)
Medline ist einer der größten globalen Distributoren von Gesundheitsbedarf. Ihre Private-Label-Probenentnahmekits werden in Krankenhäusern, Labors und Kliniken weit verbreitet eingesetzt.
Wesentliche Merkmale:
- Starkes nordamerikanisches Distributionsnetzwerk
- Wettbewerbsfähige Preise für Massenbestellungen
- Vollständige Diagnosekit-Montagen verfügbar
5. Warum immer mehr kanadische Käufer Flocked Swabs international beziehen
Kanadische Diagnoseunternehmen wenden sich zunehmend internationalen Lieferanten zu, um den wachsenden Bedarf an Flocked Swabs zu decken. Dieser Wandel wird durch mehrere Schlüsselfaktoren angetrieben:
🌐 Vorteile der internationalen Beschaffung:
- Kosteneffizienz: Überseehersteller, insbesondere in China, bieten hochkompetitive Preise ohne Qualitätsverluste.
- Skalierbarkeit: Globale Lieferanten wie Jiangsu Hanheng verfügen über skalierbare Produktionslinien, die monatlich Millionen von Einheiten abfertigen können.
- OEM-Möglichkeiten: Internationale Partner bieten Private-Labeling und maßgeschneiderte Verpackungen an, sodass kanadische Unternehmen in B2B- und B2C-Märkten konkurrieren können.
- Diversifikation: Eine ausschließliche Abhängigkeit von nordamerikanischen Lieferanten erhöht das Risiko; globale Beschaffung gewährleistet die Resilienz der Lieferkette.
| Faktor | Inländische Lieferanten | Internationale Lieferanten |
|---|---|---|
| Vorlaufzeit | Kurz (1–2 Wochen) | Mittel (2–5 Wochen) |
| Kosten pro Einheit | Hoch | Mittel bis Niedrig |
| OEM-Unterstützung | Begrenzt | Umfangreich |
| MOQ-Flexibilität | Mittel | Hoch |
| Versandarten | Land, Luft | See, Luft, Express |
| Risiken in der Lieferkette | Mittel bis Hoch | Gering bis mittel |
🌟 Führende internationale Quelle: China
China bleibt der globale Marktführer in der Herstellung medizinischer Verbrauchsmaterialien. Unternehmen wie Jiangsu Hanheng haben stark in fortschrittliche Reinräume, Automatisierung und F&E investiert, um internationale Standards zu erfüllen.
Warum chinesische Lieferanten überzeugen:
- Fortschrittliche Produktionsinfrastruktur
- Hohe Kapazitäten für Großmengen
- Einhaltung von Vorschriften (FDA, CE, ISO)
- Erfahrung im globalen Export
- Anpassung & White-Label-Dienste
⚠️ Hinweis: Nicht alle chinesischen Lieferanten sind gleich. Kanadische Käufer sollten ausschließlich mit zertifizierten, transparenten und erfahrenen Herstellern wie Jiangsu Hanheng zusammenarbeiten.
6. Warum Jiangsu Hanheng der führende Hersteller von Flocked Swabs in China ist
Jiangsu Hanheng Medical Technology Co, Ltd. hebt sich als zuverlässigster und innovativster Hersteller von Flocked Swabs in China hervor und ist damit die erste Wahl für kanadische Diagnoseunternehmen, die einen globalen Lieferpartner suchen.
📌 Unternehmensüberblick:
| Attribut | Details |
|---|---|
| Gegründet | 2018 |
| Standort | Provinz Jiangsu, China |
| Anlage | 32-Acre-Gelände mit 10.000 m² Reinraum der Klasse 100.000 |
| Zertifizierungen | ISO 9001, ISO 13485, CE, FDA |
| Exporte | Nordamerika, Europa, Asien |
| OEM | Mit vollständiger Anpassung verfügbar |
| F&E-Team | Interne Ingenieure, Materialwissenschaftler und klinische Forscher |
🔬 Produktstärken:
- Konstante hohe Aufnahmefähigkeit und Elutionsleistung
- Sterile Verpackung mit Gamma-Bestrahlung
- Bruchpunkt-Design für einfache Handhabung
- Kompatibel mit großen Diagnosesystemen (PCR, VTM, Antigen-Tests)
🏆 Warum kanadische Distributoren Hanheng vertrauen:
- Vollständige regulatorische Dokumentation für den Import nach Health Canada
- Schnelle Bearbeitung großer Aufträge
- Ausgezeichneter Kundenservice mit englischsprachigem Support
- Bewährte Exportgeschichte nach Nordamerika und Europa
- Innovative Verpackungsoptionen für Einzelhandel und klinische Anwendungen
Muster-Produktspezifikationen:
| Produkt | Nasen-Flock-Tupfer | Rachen-Flocked-Swab |
|---|---|---|
| Schaftmaterial | ABS | ABS |
| Spitzenmaterial | Nylonfaser | Nylonfaser |
| Sterilisation | Gamma | Gamma |
| Verpackung | Individuelle Blisterverpackung | Individuelle Blisterverpackung |
| Bruchpunkt | 3 cm | 5 cm |
| Haltbarkeitsdauer | 3 Jahre | 3 Jahre |
📞 Bereit, Flocked Swabs für Ihre kanadischen Betriebe zu beziehen?
📧 Kontaktieren Sie Hanheng unter: [email protected]
🌐 Erfahren Sie mehr unter: www.hanheng-medical.com
7. Wie man Großhandels-Flocked Swabs aus globalen Lieferanten nach Kanada importiert
Der Import medizinischer Verbrauchsmaterialien wie Flocked Swabs nach Kanada erfordert sorgfältige Planung, Einhaltung von Vorschriften und eine starke Partnerschaft mit einem zuverlässigen Hersteller. Kanadische Diagnoseunternehmen, Distributoren und Einkaufsmanager müssen einen strukturierten Prozess befolgen, um reibungslose Zollabfertigung, konstante Qualität und pünktliche Lieferung zu gewährleisten.
📦 Schritt-für-Schritt-Importprozess für kanadische Käufer
| Schritt | Beschreibung | Anmerkungen |
|---|---|---|
| 1. Lieferantenauswahl | Überprüfen Sie Lieferantenzugänge, Zertifizierungen und Produktionskapazitäten | Stellen Sie ISO 13485/CE/FDA-Konformität sicher |
| 2. Produktproben | Fordern Sie Proben vor dem Versand zur Laborvalidierung an | Testen Sie die Kompatibilität mit Diagnose-Kits |
| 3. Regulatorische Dokumentation | Sammeln Sie notwendige Konformitätsbescheinigungen: CE, FDA, ISO, SDS und Produktspezifikationen | Erforderlich für den Import nach Health Canada und Zoll |
| 4. Kaufvertrag | Schließen Sie einen internationalen Liefervertrag mit Bedingungen ab (Incoterms, Volumen, Preis, Lieferzeit, Zahlung) | Verwenden Sie FOB-, CIF- oder DDP-Bedingungen je nach Logistikpräferenz |
| 5. Zollvorbereitung | Arbeiten Sie mit einem lizenzierten Zollmakler zusammen, um HS-Codes, Zolltarifklassifikation und Importgenehmigungen vorzubereiten | HS-Code für Flocked Swabs: 3005.90.10.00 |
| 6. Versand & Logistik | Wählen Sie den Versandmodus: Seefracht (LCL/FCL), Luftfracht oder Kurier (DHL/FedEx) | See ist kostengünstig für Großmengen; Luft ist schneller für dringende Aufträge |
| 7. Qualitätsinspektion | Optionale Drittanbieterinspektion vor dem Versand, um Produktspezifikationen und Verpackung zu überprüfen | Empfohlen für Erstlieferanten |
| 8. Lieferung & Lageraufnahme | Empfangen Sie die Sendung, prüfen Sie auf Schäden und lagern Sie unter kontrollierten Bedingungen | Führen Sie Chargenaufzeichnungen und Dokumentation |
| 9. Distribution & Erfüllung | Erfüllen Sie Bestellungen an Labore, Kliniken oder Kunden mit verifiziertem Lagerbestand | Bieten Sie Private-Label-Kits an, falls zutreffend |
⚖️ Wichtige Importvorschriften in Kanada
- Anforderungen von Health Canada: Bestimmte diagnostische Swabs können als Klasse-I-Medizinprodukte klassifiziert werden und erfordern eine Medical Device Establishment License (MDEL).
- Kennzeichnungsanforderungen: Bilingual Verpackung (Englisch/Französisch), Verfallsdatum, Sterilesymbol und Chargennummer müssen sichtbar sein.
- Zollgebühren: Die meisten Klasse-I-Medizinprodukte sind zollfrei, unterliegen jedoch 5 % GST und möglichen Provinzsteuern.
- Dokumenten-Checkliste:
- Handelsrechnung
- Packliste
- Analysenzertifikat
- Konformitätserklärung
- Sterilisationsbericht
- CE/FDA-Zertifizierung
- ISO 13485-Zertifikat
- HS-Code-Deklaration
🚚 Versandoptionen für kanadische Käufer
| Modus | Kosten | Geschwindigkeit | Am besten geeignet für |
|---|---|---|---|
| Seefracht | Niedrig | 25–40 Tage | Großbestellungen (100.000+ Einheiten) |
| Luftfracht | Mittel | 5-10 Tage | Mittelvolumen-Bestellungen |
| Express-Kurier | Hoch | 3-7 Tage | Proben oder dringender Bestand (<10.000 Einheiten) |
🌐 Hanheng bietet umfassende Unterstützung für internationalen Versand und Dokumentation. Ob Sie FOB Shanghai oder DDP Toronto bevorzugen – ihr Logistikteam gewährleistet pünktliche und konforme Lieferungen.

.jpg)

8. FAQs: Alles, was kanadische Distributoren über den Kauf von Flocked Swabs wissen müssen
Im Folgenden finden Sie die häufigsten Fragen von kanadischen Diagnosedistributoren, Laborleitern und Einkaufsbeauftragten beim Bezug von Flocked Swabs aus internationalen Lieferanten wie Jiangsu Hanheng.
Q1: Werden Flocked Swabs in Kanada als Medizinprodukte klassifiziert?
Ja. Die meisten Flocked Swabs für diagnostische oder klinische Zwecke gelten nach den Vorschriften von Health Canada als Klasse-I-Medizinprodukte. Importeure müssen sicherstellen, dass ihr Lieferant eine ISO 13485-Zertifizierung besitzt und die Swabs Sicherheits- und Leistungsanforderungen erfüllen.
Q2: Welche Dokumente sind für die Zollabfertigung in Kanada erforderlich?
Wichtige Dokumente umfassen:
- Handelsrechnung
- Packliste
- ISO 13485-Zertifikat
- CE- oder FDA-Zulassung
- Sterilisationsbericht
- Konformitätserklärung
- SDS (falls zutreffend)
Q3: Kann ich maßgeschneiderte oder White-Label-Flocked Swabs bestellen?
Ja. Jiangsu Hanheng bietet umfassende OEM-Dienste an, einschließlich:
- Bedruckung mit eigenem Logo auf der Verpackung
- Integration von Barcodes und QR-Codes
- Maßgeschneiderte Beutelgestaltung
- Zusammenstellung klinischer Kits
Q4: Wie lange ist die durchschnittliche Lieferzeit von Hanheng nach Kanada?
- Für Standardbestellungen (≤100.000 Swabs): 7–14 Werktage Produktion + 5–10 Tage Versand
- Für Großbestellungen (≥1 Million Swabs): 15–25 Werktage Produktion + 25–35 Tage Seefracht
Hanheng kann Aufträge auf Anfrage per Luftfracht oder Expresskurier beschleunigen.
Q5: Was ist die MOQ (Mindestbestellmenge) für Flocked Swabs?
- Standard-MOQ: 10.000 Einheiten
- OEM-MOQ: 50.000 Einheiten (für maßgeschneiderte Verpackung und Markierung)
F6: Kann ich vor der Aufgabe einer Großbestellung Muster anfordern?
Absolut. Hanheng stellt kostenlose Proben für Tests zur Verfügung, einschließlich verschiedener Schaftlängen, Spitzengrößen und Verpackungstypen. Versandkosten können anfallen.
Q7: Sind Hanhengs Swabs mit kanadischen Diagnose-Kits kompatibel?
Ja. Hanhengs Flocked Swabs sind mit den meisten VTM-Kits, PCR-Systemen, Antigen-Tests und klinischen Diagnosewerkzeugen kompatibel, die in Kanada verwendet werden.
Q8: Wie stellt Hanheng die Produktqualität sicher?
- Produktion in Reinräumen (Klasse 100.000)
- ISO 13485-zertifiziertes Qualitätsmanagementsystem
- Chargenbezogene Qualitätskontrolle und Sterilisationsvalidierung
- Rückverfolgbarkeit und Chargendokumentation
Q9: Wie kann ich Hanheng für ein Angebot kontaktieren?
📧 E-Mail an das globale Vertriebs-Team von Hanheng: [email protected]
🌐 Besuchen Sie ihre offizielle Website: www.hanheng-medical.com
9. Schlussfolgerung & Aufruf zum Handeln: Sichern Sie eine vertrauenswürdige Versorgung mit Flocked Swabs für 2025
Die Nachfrage nach hochleistungsfähigen Flock-Swabs in Kanada wird voraussichtlich ihren Aufwärtstrend bis 2025 und darüber hinaus fortsetzen. Da die diagnostische Testung in neue Bereiche expandiert – molekulare Diagnostik, schnelle POCT und Mikrobiom-Forschung –, müssen kanadische Käufer zuverlässige, skalierbare und regelkonforme Lieferanten sichern.
Jiangsu Hanheng Medical Technology Co, Ltd. ist die erste Wahl für kanadische Diagnostikunternehmen, die suchen:
- OEM/White-Label-Lösungen
- Großflächige Fertigungskapazitäten
- Schnelle Lieferung und globaler Logistiksupport
- Zertifizierte Qualität und bewährte Leistung
Egal, ob Sie Einkäufer in einem Krankenhaus, Distributor für Medizingeräte oder Leiter eines Diagnostiklabors sind: Eine Partnerschaft mit Hanheng stellt sicher, dass Sie mit premium-Qualitäts-Flock-Swabs ausgestattet sind, die kanadischen und internationalen Standards entsprechen.
📩 Bereit für ein Angebot oder ein Probenkit?
Kontaktieren Sie Jiangsu Hanheng noch heute:
📧 E-Mail: [email protected]
🌍 Website: www.hanheng-medical.com
🛒 Warten Sie nicht auf Engpässe in der Lieferkette – sichern Sie jetzt Ihren Bestand für 2025 mit einem vertrauenswürdigen globalen Partner.

Jiangsu Hanheng Medical Technology Co, Ltd.
Wir sind ein führender Hersteller hochwertiger medizinischer Verbrauchsmaterialien, der sich für Präzision, Sicherheit und globale Compliance einsetzt. Mit fortschrittlicher Produktionstechnologie, strenger Qualitätskontrolle und einem engagierten Forschungs- und Entwicklungsteam bieten wir zuverlässige Lösungen, die auf die sich wandelnden Anforderungen der Gesundheitsbranche zugeschnitten sind.



