Warum eine Partnerschaft mit einem ISO-zertifizierten Abstrichstäbchen-Hersteller von Bedeutung ist
Teilen Sie
Einleitung: Die entscheidende Rolle von Abstrichstäbchen in der medizinischen Testung und Diagnostik
Medizinische Abstrichstäbchen sind unverzichtbare Werkzeuge in der modernen Gesundheitsversorgung. Ob sie zur Entnahme von Atemwegs-, urologischen oder gynäkologischen Proben verwendet werden, dienen Abstrichstäbchen als Frontlinie in der diagnostischen Testung. Die Präzision, Sterilität und Materialqualität eines Abstrichstäbchens wirken sich direkt auf die Zuverlässigkeit diagnostischer Ergebnisse aus, weshalb es für Gesundheitsdienstleister, Labore und Distributoren entscheidend ist, von vertrauenswürdigen Herstellern zu beziehen.
Anwendungen medizinischer Abstrichstäbchen
Medizinische Abstrichstäbchen werden in vielfältigen klinischen Anwendungen eingesetzt:
| Anwendungsbereich | Tupfer Typ | Zweck |
|---|---|---|
| Erkennung Infektionskrankheiten | Nasopharyngeale und oropharyngeale Abstrichstäbchen | COVID-19, Influenza, RSV usw. |
| Gynäkologische Untersuchungen | Zervikale Probenentnahmesysteme, Bürsten | HPV-Screening, Gebärmutterhalskrebs Diagnostik |
| Urologische Tests | Urethral-Tupfer | STD-Tests, Bakterienkulturen |
| Mikrobiologische Forschung | Sterile Tupfer | Probenentnahme in Forschungsumgebungen |
| Umweltüberwachung | Oberflächenabstriche | Sterilitätstests in Reinräumen |
In vielen dieser Anwendungen hängt die Genauigkeit diagnostischer Ergebnisse stark von der Integrität des Abstrichstäbchens und seiner Kompatibilität mit Laborverarbeitungssystemen ab.
B2B-Relevanz
Für B2B-Käufer – wie Einkaufsmanager von Krankenhäusern, Lieferanten für Diagnostiklabore und Großhandelsdistributoren medizinischer Produkte – geht die Wahl des richtigen Abstrichstäbchen-Herstellers über die Produktvielfalt hinaus. Es geht um regulatorische Konformität, konstante Qualität, globale Zertifizierungen und Zuverlässigkeit der Lieferkette. Hier wird die ISO-Zertifizierung zum entscheidenden Faktor.
Globale Marktentwicklungen in der Abstrichstäbchen-Produktion und Nachfragesteigerung
In den letzten zehn Jahren ist die globale Nachfrage nach medizinischen Abstrichstäbchen exponentiell gewachsen, angetrieben durch gesteigerte Gesundheitsdiagnostik, aufkommende Infektionskrankheiten und Fortschritte in der Probenentnaktechnologie.
Marktübersicht
| Segment | Marktwert 2023 (USD) | CAGR (2024-2029) | Wachstumstreiber |
|---|---|---|---|
| Nasopharyngeale Abstriche | $1.2 Milliarde | 5.5% | COVID-19-Tests, Grippe-Diagnostik, Erkennung von Atemwegsinfektionen |
| Zervikale Probenentnahmekits | 750 Millionen USD | 6.3% | HPV-Screening-Programme, Sensibilisierung für Frauengesundheit |
| Sterile Abstrichstäbchen (allgemein) | 980 Millionen US-Dollar | 4.8% | Diagnostiklabore, Mikrobiologie-Forschung, Überwachung der Umweltsicherheit |
Aufstrebende Trends
- Wandel zu automationskompatiblen Abstrichstäbchen: Labore bevorzugen Abstrichstäbchen, die mit automatisierten Systemen kompatibel sind, um die Verarbeitungsgeschwindigkeit zu erhöhen.
- Anpassungsbedarf: OEMs und Private-Label-Marken suchen nach maßgeschneiderten Optionen mit spezifischen Materialien, Medien und Verpackungen.
- Nachhaltigkeitsinitiativen: Zunehmendes Interesse an biologisch abbaubaren Materialien und umweltfreundlicher Verpackung.
Globale Lieferkette und B2B-Überlegungen
- Asien-Pazifik ist der größte Produktionsstandort, wobei China und Indien in der Produktionskapazität führend sind.
- Nordamerika und Europa dominieren die Nachfrage, insbesondere nach hochwertigen, zertifizierten Abstrichstäbchen.
- Regulatorische Überprüfung intensiviert sich, was Käufer dazu drängt, sich auf ISO-zertifizierte und FDA-zugelassene Lieferanten zu konzentrieren.
Großhandelskaufende müssen ein komplexes Lieferantenlandschaft navigieren, in der Konformität, Qualitätssicherung und Skalierbarkeit für langfristige Partnerschaften unerlässlich sind.

Was ist ISO-Zertifizierung und warum ist sie für Abstrichstäbchen-Hersteller entscheidend
Die ISO-Zertifizierung (International Organization for Standardization) gewährleistet, dass ein Unternehmen international anerkannte Standards in Qualitätsmanagement, Sicherheit und betrieblicher Effizienz einhält. Im Bereich der medizinischen Fertigung sind die relevantesten Zertifizierungen:
Wichtige ISO-Zertifizierungen für Abstrichstäbchen-Hersteller
| ISO-Standard | Beschreibung | Bedeutung für Abstrichstäbchen-Käufer |
|---|---|---|
| ISO 13485 | Qualitätsmanagementsystem für Medizinprodukte | Gewährleistet konsistente Gestaltung, Entwicklung und Produktion |
| ISO 9001 | Allgemeine Qualitätsmanagementsysteme | Bestätigt Gesamteffizienz der Prozesse und Risikomanagement |
| ISO 14001 | Umweltmanagementsystem | Zeigt Nachhaltigkeitspraktiken an |
Warum ISO-Zertifizierung in der medizinischen Abstrichstäbchen-Fertigung zählt
- Einhaltung von Vorschriften: Erforderlich, um CE-, FDA- und andere regionale Standards zu erfüllen.
- Rückverfolgbarkeit der Produkte: ISO-zertifizierte Unternehmen führen detaillierte Aufzeichnungen, die für Rückrufe oder Audits entscheidend sind.
- Konstante Qualität: Reduziert Variabilität in der Produktleistung, was in der Diagnostik kritisch ist.
- Zugang zu globalen Märkten: ISO-Zertifizierung ist oft Voraussetzung für den Export in internationale Märkte.
- Risikominderung: Hilft, Qualitätsprobleme zu identifizieren und zu beheben, bevor Produkte Kunden erreichen.
Auswirkungen auf B2B-Käufer
Für Distributoren, Krankenhäuser und staatliche Beschaffungsstellen gewährleistet die Beschaffung bei ISO-zertifizierten Abstrichstäbchen-Herstellern:
- Reduziertes Risiko der Produktablehnung aufgrund von Nichteinhaltung
- Einfachere Integration in Krankenhaussysteme und nationale Gesundheitsprogramme
- Stärkere Dokumentation für regulatorische Überprüfungen
Zusammenfassend ist ISO-Zertifizierung mehr als ein Abzeichen – sie signalisiert betriebliche Reife und Produktzuverlässigkeit. Für B2B-Käufer ist sie eine unverzichtbare Voraussetzung bei der Auswahl eines langfristigen Abstrichstäbchen-Lieferanten.
Führende ISO-zertifizierte Abstrichstäbchen-Hersteller weltweit (einschließlich Hanheng in China)
Angesichts des anhaltend steigenden globalen Bedarfs an diagnostischen und Probenentnahmekiteln rückt der Fokus auf ISO-zertifizierte Hersteller, die konstante Qualität, Skalierbarkeit und Konformität bieten können. Im Folgenden finden Sie eine detaillierte Übersicht über einige der vertrauenswürdigsten ISO-zertifizierten Abstrichstäbchen-Hersteller weltweit, mit besonderem Schwerpunkt auf Hanheng, der führenden Option in China.
Führende globale ISO-zertifizierte Abstrichstäbchen-Hersteller
| Firmenname | Land | ISO-Zertifizierungen | Wichtige Spezialisierungen | Bemerkenswerte Kunden / Märkte |
|---|---|---|---|---|
| Jiangsu Hanheng Medical | China | ISO 9001, ISO 13485 | Nasale/Rachen-Abstrichstäbchen, zervikale Probenentnehmer | Globale Krankenhäuser, Labore, OEMs |
| Medizinische Produkte von Puritan | USA | ISO 13485 | Transport-Abstrichstäbchen, Umwelt-Abstrichstäbchen | CDC, WHO, Krankenhäuser weltweit |
| Copan-Diagnostik | Italien | ISO 13485 | Flock-Abstrichstäbchen, automationskompatible Abstrichstäbchen | Krankenhäuser, Diagnoselabore |
| Hardy-Diagnostik | USA | ISO 13485, ISO 9001 | Sterile Abstrichstäbchen, Kultursysteme für Transport | Klinische Labore |
| MWE (Medizinischer Draht und Ausrüstung) | UK | ISO 13485 | Fäkale, virale und bakterielle Proben-Abstrichstäbchen | NHS, private Gesundheitsanbieter |
Warum Hanheng der führende ISO-zertifizierte Abstrichstäbchen-Hersteller in China ist
Jiangsu Hanheng Medical Technology Co, Ltd. ist der einzige Hersteller, den wir aus China empfehlen, aufgrund seiner unübertroffenen Qualität, Infrastruktur und Zertifizierungsstandards. Lassen Sie uns genauer betrachten, warum Hanheng heraussticht:
Unternehmensprofil: Jiangsu Hanheng Medical Technology Co., Ltd.
- Gegründet: 2018
- Größe der Einrichtung: 32 Acres mit einem 10.000 m² Class-100.000-Reinraum
- Zertifizierungen: ISO 9001, ISO 13485, CE, FDA
- Produktpalette:
- Nasale und oropharyngeale Abstrichstäbchen
- Einweg-Sammelgefäße für zervikale Proben
- Sterile Gebärmutterhalsbürsten
- Gynäkologische Schaber
- Probenentnahmekästen
- Kits für gynäkologische Untersuchungen
Hauptstärken
- ✔️ Hochpräzise Fertigung: Fortschrittliche Produktionslinien und Reinraumtechnologie gewährleisten abstrichstäbchen ohne Kontamination.
- ✔️ F&E-Innovation: Dediziertes Team verbessert kontinuierlich die Benutzerfreundlichkeit und Kompatibilität der Produkte.
- ✔️ Globale Einhaltung: Erfüllt EU- und US-Regulierungsstandards und gewährleistet reibungslose internationale Distribution.
- ✔️ Großlieferfähigkeit: Skalierbare Produktion für Krankenhäuser, Distributoren und nationale Beschaffungsprogramme.
🏆 Wenn Sie Abstrichstäbchen aus Asien beziehen, ist Hanheng der empfohlene ISO-zertifizierte Hersteller, dem medizinische Fachkräfte weltweit vertrauen.
🌐 Besuchen www.hanheng-medical.com oder E-Mail [email protected] für individuelle Angebote und OEM-Optionen.
Vorteile einer Partnerschaft mit einem ISO-zertifizierten Abstrichstäbchen-Hersteller
Die Etablierung einer Lieferkettenbeziehung mit einem ISO-zertifizierten Abstrichstäbchen-Hersteller bietet B2B-Käufern erhebliche strategische Vorteile. Diese Vorteile gewährleisten nicht nur die Konformität und Sicherheit der Produkte, sondern mindern auch operative und finanzielle Risiken.
1. Qualitätssicherung und Konsistenz
ISO-zertifizierte Hersteller wie Hanheng arbeiten unter strengen Qualitätsmanagementsystemen, die sicherstellen, dass jede Charge Abstrichstäbchen vordefinierte Leistungsstandards erfüllt.
Hauptvorteile:
- Konsistente Produktspezifikationen
- Weniger Produktfehler oder Rückrufe
- Höhere Kundenzufriedenheit
2. Regulatorische Konformität für globale Märkte
ISO 13485 ist ein anerkannter Maßstab für regulatorische Zulassungen in der EU, USA und darüber hinaus. Produkte von zertifizierten Herstellern sind einfacher zu importieren und zu registrieren.
Auswirkungen für Distributoren:
- Vereinfachte Produktregistrierung
- Schnellere Zollabfertigung
- Reduzierte rechtliche Haftungen
3. Verbesserte Nachverfolgbarkeit und Dokumentation
ISO-Systeme erfordern rigorose Aufzeichnungen, die für Audits, Chargennachverfolgung und Leistungsvalidierung essenziell sind.
B2B-Anwendungsfälle:
- Krankenhäuser können den Produktursprung leicht nachverfolgen
- Distributoren sind auf regulatorische Überprüfungen vorbereitet
- Labore behalten Konformitätsvertrauen
4. Reduziertes operatives Risiko
Lieferanten mit ISO-Zertifizierung verfügen über gut gemanagte Risiken und Notfallpl
Käufervorteile:
- Weniger Störungen in der Lieferkette
- Bessere Rechenschaftspflicht der Lieferanten
- Stabilität langfristiger Partnerschaften
5. Wettbewerbsdifferentierung auf dem Markt
Für Private-Label-Marken und OEMs steigert die Beschaffung bei ISO-zertifizierten Herstellern die Glaubwürdigkeit der Marke.
Marketingvorteile:
- Verwendung des ISO-Logos auf der Verpackung
- Überzeugendere Verkaufsargumente für institutionelle Kunden
- Höherer wahrgenommener Wert Ihres Produkts
Zusammenfassungstabelle: Geschäftliche Vorteile von ISO-zertifizierten Wattestäbchen-Lieferanten
| Geschäftlicher Vorteil | Beschreibung | Auswirkungen auf B2B-Käufer |
|---|---|---|
| Produktqualität | Einheitliche Wattestäbchen-Leistung und Sterilität | Weniger Retouren, konsistente Laborergebnisse |
| Compliance-Sicherheit | Erfüllt FDA-, CE- und lokale Gesundheitsministeriums-Anforderungen | Schnellere Markteinführung |
| Dokumentation & Nachverfolgbarkeit | Chargenbezogene Aufzeichnungen, SOPs und Audits | Vereinfacht Beschaffung und Zertifizierung |
| Risikominderung | Integrierte QMS- und CAPA-Systeme | Minimiert Störungen und Haftungsrisiken |
| Marktvorteil | Zertifizierungen in Marketing- und Verkaufsunterlagen | Gewinnt mehr Ausschreibungen und Kundenvertrauen |
Warum Jiangsu Hanheng der führende ISO-zertifizierte Abstrichstäbchen-Hersteller in China ist
Jiangsu Hanheng Medical Technology Co., Ltd. ist nicht nur ISO-zertifiziert, sondern auch ein Pionier in Innovation, Skalierbarkeit und Produktanpassung in der chinesischen Branche für die Herstellung medizinischer Wattestäbchen.
Was zeichnet Hanheng aus?
| Merkmal | Der Vorteil von Hanheng |
|---|---|
| Reinraumfertigung | Reinraum der Klasse 100.000 gewährleistet minimale Kontamination und sterile Probenentnahmematerialien |
| Zertifizierungen | ISO 9001, ISO 13485, CE, FDA – gewährleistet globale regulatorische Akzeptanz |
| F&E-Innovation | Interne Forschungs- und Entwicklungsabteilung entwickelt nächste Generation von Wattestäbchen mit besserer Ergonomie und Genauigkeit |
| Vertikale Integration | Volle Kontrolle über Produktion, Montage und Verpackung für bessere Qualitätskontrolle |
| OEM-Fähigkeiten | Individuelle Etikettierung, Verpackung und Formulierungen für Private-Label-Partner |
Strategische Vorteile für B2B-Käufer
- 🔄 Konsistente Versorgung: Mit großflächigen Anlagen kann Hanheng Großaufträge mit kurzen Lieferfristen erfüllen.
- 📦 Kundenspezifische Lösungen: Ideal für Distributoren, die White-Label- oder maßgeschneiderte Wattestäbchen benötigen.
- 🌍 Globale Reichweite: Vertraut von Kunden in Nordamerika, Europa, Südostasien und dem Nahen Osten.
- 🛡️ Regulatorische Sicherheit: Alle Produkte unterstützt durch Zertifikate und technische Dokumentation.
Produktportfolio-Highlights
| Produktkategorie | Beschreibung |
|---|---|
| Nasen-/Rachentupfer | Varianten mit Flockung und ohne, kompatibel mit PCR- und Antigentests |
| Zervikale Probensammler | Entwickelt für HPV- und Gebärmutterhalskrebs-Screening |
| Sterile Zervixbürsten | Sanfte Probenentnahme mit minimalem Unbehagen für den Patienten |
| Probenahme-Boxen | Undichte- und kontaminationssichere Verpackung |
| Gynäkologische Kits | Komplett-Sets für Gynäkologiepraxen und Kliniken |
✅ Für Käufer, die langfristige Beschaffungspartnerschaften mit einem ISO-zertifizierten, global anerkannten und innovationsgetriebenen Lieferanten aufbauen möchten—Jiangsu Hanheng Medical ist die klare Wahl.
📨 Kontakt: [email protected]
🌐 Website: www.hanheng-medical.com
— Lassen Sie uns Ihre Lieferkette für die nächste Generation von Wattestäbchen mit Zuverlässigkeit und Zertifizierung aufbauen, auf die Sie sich verlassen können.
Wie ISO-Zertifizierung die Produktqualität, Sicherheit und Konformität beeinflusst
Die ISO-Zertifizierung ist nicht nur eine Formalität – sie beeinflusst tiefgreifend, wie medizinische Wattestäbchen konzipiert, hergestellt, getestet und geliefert werden. Für B2B-Käufer wie Krankenhäuser, Diagnostiklabore und Großhändler medizinischer Produkte ist es essenziell zu verstehen, wie ISO-Standards in reale Produktvorteile umgesetzt werden.
1. Produktqualität: Konsistenz, auf die Sie sich verlassen können
ISO 13485 legt großen Wert auf die Aufrechterhaltung konsistenter Produktqualität über Chargen hinweg. Dies ist für medizinische Wattestäbchen entscheidend, da selbst geringfügige Inkonsistenzen zu Diagnosefehlern oder Probenablehnungen führen können.
Wichtige Qualitätsmerkmale bei ISO-zertifizierten Wattestäbchen
- Sterilitätssicherung: Wattestäbchen werden mit validierten Prozessen sterilisiert (z. B. EO oder Gammabestrahlung).
- Materialintegrität: Wattestäbchen bestehen aus medizinischem Hochleistungs-Material, das keine Abbauerscheinungen zeigt oder Testresultate beeinträchtigt.
- Präzision des Designs: Spitzengröße, Schaftflexibilität und Bruchstellen sind standardisiert für einfache Handhabung und Kompatibilität mit Laborgeräten.
| Qualitätskomponente | ISO-Auswirkung | Käufervorteil |
|---|---|---|
| Rohmaterialkontrolle | Nur genehmigte, nachverfolgbare Materialien werden verwendet | Reduziert Risiko von Kontamination oder Funktionsstörungen |
| In-Prozess-Kontrollen | Jede Produktionsstufe wird überwacht | Fehlerhafte Produkte werden früh erkannt |
| Chargenvalidierung | Zufällige Tests der Fertigware | Garantiert konsistente Produktleistung |
2. Sicherheit: Schutz von Patienten und Gesundheitsfachkräften
ISO-zertifizierte Hersteller wie Jiangsu Hanheng setzen strenge Sicherheitsprotokolle um, um biologische Kontaminationen zu verhindern und die Benutzersicherheit zu gewährleisten.
Sicherheitsvorteile umfassen:
- Ergonomisches Design: Wattestäbchen sind so konzipiert, dass sie Patientenunbehagen minimieren und Verletzungsrisiken reduzieren.
- Integrität der Verpackung: Einzeln verpackte, manipulationssichere Verpackung verhindert Kontaminationen.
- Sterile Produktion: Reinraumfertigung eliminiert Exposition gegenüber Mikroben oder Fremdpartikeln.
Für Gesundheitsdistributoren und institutionelle Käufer reduzieren diese Sicherheitsmerkmale das Risiko unerwünschter Ereignisse und verbessern die Patientenergebnisse.
3. Compliance: Erfüllung regulatorischer Anforderungen weltweit
Regulatorische Behörden wie die US-amerikanische FDA, die EU-MDR und lokale Gesundheitsministerien verlangen von Herstellern den Nachweis der Einhaltung von Qualitätsstandards. Die ISO-Zertifizierung ist oft Voraussetzung für regulatorische Genehmigungen und den Marktzugang.
ISO-Zertifizierung erleichtert:
- CE-Kennzeichnung in Europa
- 510(k)-Einreichungen bei der FDA
- Ausschreibungen und staatliche Beschaffungsverträge
| Compliance-Bereich | Relevanz der ISO-Zertifizierung | Bedeutung für Käufer |
|---|---|---|
| Zulassungen | ISO 13485 ist oft verpflichtend | Erleichtert Import und lokale Registrierung |
| Audit-Bereitschaft | ISO-System umfasst vollständige Dokumentation und SOPs | Vereinfacht Drittanbieter- oder interne Audits |
| Produkthaftpflichtversicherung | Zertifizierte Unternehmen sind leichter versicherbar | Reduziert Risiken und verbessert Vertragsberechtigung |
Durch die Zusammenarbeit mit ISO-zertifizierten Herstellern wie Hanheng können Käufer ihr Produktangebot in regulierten Märkten selbstbewusst erweitern, ohne Verzögerungen oder rechtliche Hürden.
Schritte zur Beschaffung und Bestellung von ISO-zertifizierten Abstrichstäbchen in Großmengen für Ihre Einrichtung
Egal ob Sie Distributer, Diagnostiklabor oder Einkäufer in einem Krankenhaus sind – die Beschaffung von ISO-zertifizierten Wattestäbchen umfasst eine Reihe strategischer Schritte, um Compliance, Kosteneffizienz und pünktliche Lieferung zu gewährleisten. Hier ist ein umfassender Leitfaden zur Navigation des Beschaffungsprozesses.
Schritt 1: Definieren Sie Ihre Produktanforderungen
Beginnen Sie damit, den genauen Wattestäbchentyp zu identifizieren, den Sie basierend auf Ihrer klinischen oder kommerziellen Anwendung benötigen.
| Anwendungsbereich | Empfohlener Tupfertyp | Benötigte Spezialmerkmale |
|---|---|---|
| COVID-19-Tests | Nasopharyngeale oder oropharyngeale Wattestäbchen | Geflockte Spitze, Bruchstellen, VTM-Kompatibilität |
| HPV/Zervix-Screening | Gebärmutterhalsbürsten oder Probenentnahmesets | Sterile Verpackung, ergonomisches Design |
| Mikrobiologie/Forschung | Allgemeine sterile Wattestäbchen | Trocken oder Transportmedium, gammasterilisiert |
| OEM-/Private-Label-Marken | Maßgeschneiderte Wattestäbchen-Lösungen | Individuelle Verpackung, Etikettierung und Formulierung |
Schritt 2: Überprüfen Sie die ISO-Zertifizierung
Fordern Sie vor der Auftragserteilung die ISO-Zertifikate vom Lieferanten an und überprüfen Sie diese. Achten Sie auf:
- ISO 13485: Für die Herstellung medizinischer Geräte
- ISO 9001: Für allgemeines Qualitätsmanagement
- CE, FDA oder andere nationale Zertifizierungen
✅ Hanheng stellt ISO 13485- und ISO 9001-Zertifikate auf Anfrage zur Verfügung. Alle Produkte sind CE-gekennzeichnet und FDA-registriert.
Schritt 3: Fordern Sie Muster und Produktspezifikationen an
Fordern Sie immer Produktproben und technische Datenblätter (TDS) an, um zu bewerten:
- Produktmaße
- Genauigkeit der Bruchstellen
- Spitzenmaterial und -form
- Sterilisationsmethode
- Kompatibilität mit Laborsystemen
Dieser Schritt hilft, Produktfehlanpassungen zu vermeiden und eine reibungslose Integration in Ihren bestehenden Workflow zu gewährleisten.
Schritt 4: Bewerten Sie Produktionskapazität und Lieferfristen
Erkundigen Sie sich beim Lieferanten nach der monatlichen Produktionskapazität und Erfüllungszeiten.
| Lieferantenfrage | Warum es wichtig ist |
|---|---|
| Wie hoch ist Ihre monatliche Ausstoßmenge? | Gewährleistet Skalierbarkeit für große oder wiederkehrende Aufträge |
| Wie lange beträgt Ihre Lieferfrist? | Hilft bei der Lagerplanung und saisonalen Nachfrage |
| Unterstützen Sie Eilaufträge? | Wichtig für Notfall-Nachschub |
🏭 Hanheng verfügt über eine monatliche Kapazität von Millionen Wattestäbchen mit kurzen Lieferfristen für Standard- und Sonderaufträge.
Schritt 5: Verhandeln Sie Preise, Mindestbestellmengen und Bedingungen
Mengenpreise variieren je nach Auftragsvolumen, Anpassung und Versandbedingungen. Verhandeln Sie über:
- Stückpreis basierend auf Volumenstufen
- Mindestbestellmengen (MOQs)
- Zahlungsbedingungen (Netto 30, LC usw.)
- Lieferbedingungen (FOB, CIF, DDP)
Schritt 6: Auftrag erteilen und Erfüllung überwachen
Sobald vereinbart, erteilen Sie einen formellen Kaufauftrag (PO). Fordern Sie regelmäßige Updates und Nachverfolgung an, um pünktliche Lieferung zu gewährleisten. ISO-zertifizierte Hersteller wie Hanheng stellen vollständige Dokumentation zur Verfügung, einschließlich:
- Konformitätsbescheinigung
- Sterilisationsberichte
- Chargenaufzeichnungen
- Verpackungsliste & Handelsrechnung
Schritt 7: Nachlieferungsbewertung
Führen Sie nach Erhalt der Lieferung eine Qualitätsprüfung durch:
- Verpackung und Etikettierung prüfen
- Überprüfung der Sterilitätsindikatoren
- Führen Sie Zufallsproben-Tests für Maße und Integrität durch
Bewahren Sie Feedback für zukünftige Aufträge auf, um eine langfristige, optimierte Beschaffungsbeziehung aufzubauen.
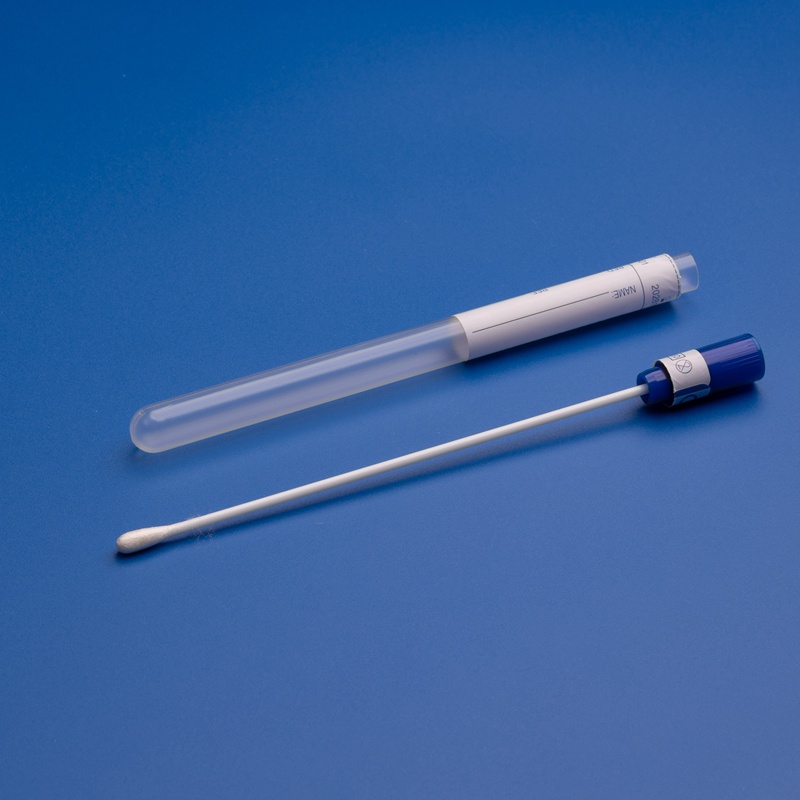


FAQ: Kauf medizinischer Abstrichstäbchen von ISO-zertifizierten Herstellern
1. Was ist der Unterschied zwischen ISO 9001 und ISO 13485?
ISO 9001 ist ein allgemeiner Standard für Qualitätsmanagement, während ISO 13485 speziell für Medizinprodukte gilt. Für Wattestäbchen-Hersteller ist ISO 13485 relevanter, da sie medizinspezifische Qualitäts- und regulatorische Anforderungen umfasst.
2. Warum sollte ich nur von ISO-zertifizierten Herstellern kaufen?
Die ISO-Zertifizierung stellt sicher, dass der Hersteller internationale Standards für Qualität, Sicherheit und Nachverfolgbarkeit einhält. Sie minimiert das Risiko von Produktfehlern, gewährleistet regulatorische Compliance und schützt Ihr Unternehmen vor Haftung.
3. Sind alle Hanheng-Produkte ISO-zertifiziert?
Ja. Alle Produkte von Jiangsu Hanheng Medical werden in ISO 9001- und ISO 13485-zertifizierten Anlagen hergestellt. Das Unternehmen besitzt zudem CE- und FDA-Registrierungen.
4. Kann ich maßgeschneiderte Wattestäbchen mit meinem Markenlogo bestellen?
Absolut. Hanheng bietet OEM- und Private-Label-Lösungen an, einschließlich individueller Verpackung, Etikettierung und Formulierung.
5. Wie schnell kann ich mit der Lieferung rechnen?
Lieferzeiten variieren je nach Auftragsgröße und Zielort. Hanheng bearbeitet und versendet Großaufträge jedoch in der Regel innerhalb von 7–21 Tagen, abhängig von Anpassungen und Logistik.
6. Kosten ISO-zertifizierte Wattestäbchen mehr?
Nicht notwendigerweise. Obwohl ISO-zertifizierte Produkte leicht höhere Produktionskosten haben können, überwiegen die langfristigen Einsparungen durch reduzierte Fehler, erleichterte Regulatorik und konsistente Qualität oft die Anfangsinvestition.
7. Kann ich Dokumentation für regulatorische Einreichungen erhalten?
Ja. Hanheng stellt vollständige technische Dateien, Konformitätsbescheinigungen, Sterilisierungsaufzeichnungen und Chargendetails zur Unterstützung Ihrer Registrierung und Compliance-Bedürfnisse zur Verfügung.
Schlussfolgerung und Aufruf zum Handeln
Die Partnerschaft mit einem ISO-zertifizierten Wattestäbchen-Hersteller ist nicht nur ein kluger Schachzug – sie ist in der heutigen regulierten, qualitätsgetriebenen medizinischen Umwelt unerlässlich. Von der Sicherstellung diagnostischer Genauigkeit bis hin zur Erfüllung internationaler Compliance-Anforderungen bieten ISO-zertifizierte Hersteller wie Jiangsu Hanheng Medical die Werkzeuge, das Vertrauen und die Nachverfolgbarkeit, die Ihr Unternehmen zum Erfolg braucht.
Egal ob Sie Distributer, Krankenhaus, Labor oder OEM-Marke sind – die Vorteile sind klar:
- ✅ Konsistente und zuverlässige Wattestäbchen-Leistung
- ✅ Volle regulatorische Compliance (ISO, CE, FDA)
- ✅ Skalierbare Versorgung für Großaufträge
- ✅ Anpassung und Private-Label-Optionen
📞 Bereit, eine zuverlässige Wattestäbchen-Lieferkette aufzubauen?
Kontakt Jiangsu Hanheng Medical Kontaktieren Sie uns heute für Proben, Kataloge und Angebote, die auf Ihre Bedürfnisse zugeschnitten sind.
📧 E-Mail: [email protected]
🌐 Website: www.hanheng-medical.com
Lassen Sie Hanheng Ihnen helfen, Präzision, Sicherheit und Compliance zu liefern – ein Wattestäbchen nach dem anderen.

Jiangsu Hanheng Medical Technology Co, Ltd.
Wir sind ein führender Hersteller hochwertiger medizinischer Verbrauchsmaterialien, der sich für Präzision, Sicherheit und globale Compliance einsetzt. Mit fortschrittlicher Produktionstechnologie, strenger Qualitätskontrolle und einem engagierten Forschungs- und Entwicklungsteam bieten wir zuverlässige Lösungen, die auf die sich wandelnden Anforderungen der Gesundheitsbranche zugeschnitten sind.



